Improvements in health-related quality of life after treatment with tocilizumab in patients with rheumatoid arthritis refractory to tumour necrosis factor inhibitors: results from the 24-week randomized controlled RADIATE study
- PMID: 22753773
- PMCID: PMC3448882
- DOI: 10.1093/rheumatology/kes131
Improvements in health-related quality of life after treatment with tocilizumab in patients with rheumatoid arthritis refractory to tumour necrosis factor inhibitors: results from the 24-week randomized controlled RADIATE study
Abstract
Objective: To investigate the effect of tocilizumab on patient-reported outcomes (PROs) in RA patients with inadequate responses to TNF inhibitors (TNFis).
Methods: In a Phase III randomized controlled trial, 489 patients received 4 or 8 mg/kg tocilizumab or placebo every 4 weeks plus MTX for 24 weeks. Mean changes from baseline over time and proportions of patients reporting improvements greater than or equal to minimum clinically important differences (MCIDs) in PROs were analyzed.
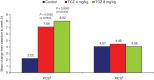
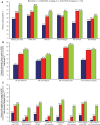
Results: At week 24, 8 mg/kg resulted in significantly greater improvements vs placebo in pain, global assessment of disease activity (P=0.001), Health Assessment Questionnaire-Disability Index (HAQ-DI; P<0.0001), Functional Assessment of Chronic Illness Therapy-Fatigue (P=0.0150) and Medical Outcomes Survey Short Form 36 (SF-36 v2) Physical Component Summary (PCS; P=0.0003) scores, all greater than MCID; 4 mg/kg resulted in greater improvements in pain (P=0.0100), HAQ-DI (P=0.0030) and SF-36 PCS (P = 0.0020) scores. Tocilizumab-associated improvements were evident as early as week 2. At week 24, more tocilizumab-treated than control patients reported improvements greater than or equal to MCID in SF-36 domain scores and related PROs (50.9-84.9% vs 35.0-51.7%) and achieved ACR50 responses and/or Disease Activity Score 28 (DAS28) remission with PRO improvements greater than or equal to MCID (36.2-51.2% vs 10-20.7% and 10.7-37.5% vs 0.0-3.4%, respectively).
Conclusion: Tocilizumab treatment in patients with inadequate responses to TNFis resulted in rapid and sustained improvements in multiple PROs that were statistically significant and clinically meaningful, consistent with previous efficacy reports. Trial Registration. ClinicalTrials.gov, http://clinicaltrials.gov/, NCT00106522.
Figures


References
-
- Lubeck DP. Health-related quality of life measurements and studies in rheumatoid arthritis. Am J Manag Care. 2002;8:811–20. - PubMed
-
- Massarotti EM. Clinical and patient-reported outcomes in clinical trials of abatacept in the treatment of rheumatoid arthritis. Clin Ther. 2008;30:429–42. - PubMed
-
- Khanna D, Tsevat J. Health-related quality of life—an introduction. Am J Manag Care. 2007;13:S218–23. - PubMed
-
- Strand V, Singh JA. Newer biological agents in rheumatoid arthritis: impact on health-related quality of life and productivity. Drugs. 2010;70:121–45. - PubMed
-
- Kirwan JR, Minnock P, Adebajo A, et al. Patient perspective: fatigue as a recommended patient centered outcome measure in rheumatoid arthritis. J Rheumatol. 2007;34:1174–7. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

