Stem and progenitor cells in myelodysplastic syndromes show aberrant stage-specific expansion and harbor genetic and epigenetic alterations
- PMID: 22753872
- PMCID: PMC3437595
- DOI: 10.1182/blood-2011-12-399683
Stem and progenitor cells in myelodysplastic syndromes show aberrant stage-specific expansion and harbor genetic and epigenetic alterations
Abstract
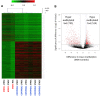
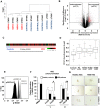
Even though hematopoietic stem cell (HSC) dysfunction is presumed in myelodysplastic syndrome (MDS), the exact nature of quantitative and qualitative alterations is unknown. We conducted a study of phenotypic and molecular alterations in highly fractionated stem and progenitor populations in a variety of MDS subtypes. We observed an expansion of the phenotypically primitive long-term HSCs (lineage(-)/CD34(+)/CD38(-)/CD90(+)) in MDS, which was most pronounced in higher-risk cases. These MDS HSCs demonstrated dysplastic clonogenic activity. Examination of progenitors revealed that lower-risk MDS is characterized by expansion of phenotypic common myeloid progenitors, whereas higher-risk cases revealed expansion of granulocyte-monocyte progenitors. Genome-wide analysis of sorted MDS HSCs revealed widespread methylomic and transcriptomic alterations. STAT3 was an aberrantly hypomethylated and overexpressed target that was validated in an independent cohort and found to be functionally relevant in MDS HSCs. FISH analysis demonstrated that a very high percentage of MDS HSC (92% ± 4%) carry cytogenetic abnormalities. Longitudinal analysis in a patient treated with 5-azacytidine revealed that karyotypically abnormal HSCs persist even during complete morphologic remission and that expansion of clonotypic HSCs precedes clinical relapse. This study demonstrates that stem and progenitor cells in MDS are characterized by stage-specific expansions and contain epigenetic and genetic alterations.
Figures



Comment in
-
MDS: roadblock to differentiation.Blood. 2012 Sep 6;120(10):1968-9. doi: 10.1182/blood-2012-07-442749. Blood. 2012. PMID: 22956526 No abstract available.
References
-
- Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med. 2006;355(12):1253–1261. - PubMed
-
- Jamieson CH, Ailles LE, Dylla SJ, et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N Engl J Med. 2004;351(7):657–667. - PubMed
-
- Tehranchi R, Woll PS, Anderson K, et al. Persistent malignant stem cells in del(5q) myelodysplasia in remission. N Engl J Med. 2010;363(11):1025–1037. - PubMed
-
- Nilsson L, Eden P, Olsson E, et al. The molecular signature of MDS stem cells supports a stem-cell origin of 5q myelodysplastic syndromes. Blood. 2007;110(8):3005–3014. - PubMed
-
- Nilsson L, Astrand-Grundstrom I, Arvidsson I, et al. Isolation and characterization of hematopoietic progenitor/stem cells in 5q−deleted myelodysplastic syndromes: evidence for involvement at the hematopoietic stem cell level. Blood. 2000;96(6):2012–2021. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

