Bnip3 and AIF cooperate to induce apoptosis and cavitation during epithelial morphogenesis
- PMID: 22753893
- PMCID: PMC3392936
- DOI: 10.1083/jcb.201111063
Bnip3 and AIF cooperate to induce apoptosis and cavitation during epithelial morphogenesis
Erratum in
- J Cell Biol. 2013 Apr 1;201(1):165
Abstract
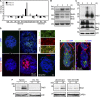
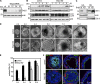
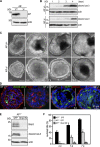
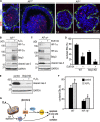
Apoptosis is an essential step in cavitation during embryonic epithelial morphogenesis, but its mechanisms are largely unknown. In this paper, we used embryonic stem cell-differentiated embryoid bodies (EBs) as a model and found that Bnip3 (Bcl-2/adenovirus E1B 19-kD interacting protein), a BH3-only proapoptotic protein, was highly up-regulated during cavitation in a hypoxia-dependent manner. Short hairpin RNA silencing of Bnip3 inhibited apoptosis of the core cells and delayed cavitation. We show that the Bnip3 up-regulation was mediated mainly by hypoxia-inducible factor (HIF)-2. Ablation of HIF-2α or HIF-1β, the common β subunit of HIF-1 and -2, suppressed Bnip3 up-regulation and inhibited apoptosis and cavitation. We further show that apoptosis-inducing factor (AIF) cooperated with Bnip3 to promote lumen clearance. Bnip3 silencing in AIF-null EBs nearly blocked apoptosis and cavitation. Moreover, AIF also regulated Bnip3 expression through mitochondrial production of reactive oxygen species and consequent HIF-2α stabilization. These results uncover a mechanism of cavitation through hypoxia-induced apoptosis of the core cells mediated by HIFs, Bnip3, and AIF.
Figures



Similar articles
-
PTEN induces apoptosis and cavitation via HIF-2-dependent Bnip3 upregulation during epithelial lumen formation.Cell Death Differ. 2015 May;22(5):875-84. doi: 10.1038/cdd.2014.185. Epub 2014 Nov 14. Cell Death Differ. 2015. PMID: 25394489 Free PMC article.
-
Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains.Mol Cell Biol. 2009 May;29(10):2570-81. doi: 10.1128/MCB.00166-09. Epub 2009 Mar 9. Mol Cell Biol. 2009. PMID: 19273585 Free PMC article.
-
Testosterone induces renal tubular epithelial cell death through the HIF-1α/BNIP3 pathway.J Transl Med. 2019 Feb 28;17(1):62. doi: 10.1186/s12967-019-1821-7. J Transl Med. 2019. PMID: 30819186 Free PMC article.
-
Apoptosis-inducing factor (AIF): a novel caspase-independent death effector released from mitochondria.Biochimie. 2002 Feb-Mar;84(2-3):215-22. doi: 10.1016/s0300-9084(02)01374-3. Biochimie. 2002. PMID: 12022952 Review.
-
The role of the hypoxia-inducible BH3-only proteins BNIP3 and BNIP3L in cancer.Cancer Metastasis Rev. 2007 Dec;26(3-4):553-66. doi: 10.1007/s10555-007-9080-0. Cancer Metastasis Rev. 2007. PMID: 17805942 Review.
Cited by
-
Hypoxia potentiates the cytotoxic effect of piperlongumine in pheochromocytoma models.Oncotarget. 2016 Jun 28;7(26):40531-40545. doi: 10.18632/oncotarget.9643. Oncotarget. 2016. PMID: 27244895 Free PMC article.
-
NIPAAm-MMA nanoparticle-encapsulated visnagin ameliorates myocardial ischemia/reperfusion injury through the promotion of autophagy and the inhibition of apoptosis.Oncol Lett. 2018 Apr;15(4):4827-4836. doi: 10.3892/ol.2018.7922. Epub 2018 Feb 1. Oncol Lett. 2018. PMID: 29552122 Free PMC article.
-
Caspase-Dependent and Caspase-Independent Pathways Are Involved in Cadmium-Induced Apoptosis in Primary Rat Proximal Tubular Cell Culture.PLoS One. 2016 Nov 18;11(11):e0166823. doi: 10.1371/journal.pone.0166823. eCollection 2016. PLoS One. 2016. PMID: 27861627 Free PMC article.
-
Cap-independent translation by DAP5 controls cell fate decisions in human embryonic stem cells.Genes Dev. 2016 Sep 1;30(17):1991-2004. doi: 10.1101/gad.285239.116. Genes Dev. 2016. PMID: 27664238 Free PMC article.
-
BNIP3 induction by hypoxia stimulates FASN-dependent free fatty acid production enhancing therapeutic potential of umbilical cord blood-derived human mesenchymal stem cells.Redox Biol. 2017 Oct;13:426-443. doi: 10.1016/j.redox.2017.07.004. Epub 2017 Jul 4. Redox Biol. 2017. PMID: 28704726 Free PMC article.
References
-
- Brown D., Yu B.D., Joza N., Bénit P., Meneses J., Firpo M., Rustin P., Penninger J.M., Martin G.R. 2006. Loss of Aif function causes cell death in the mouse embryo, but the temporal progression of patterning is normal. Proc. Natl. Acad. Sci. USA. 103:9918–9923 10.1073/pnas.0603950103 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

