Z-line formins promote contractile lattice growth and maintenance in striated muscles of C. elegans
- PMID: 22753896
- PMCID: PMC3392944
- DOI: 10.1083/jcb.201202053
Z-line formins promote contractile lattice growth and maintenance in striated muscles of C. elegans
Abstract
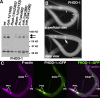
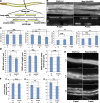
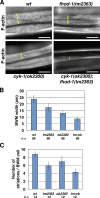
Muscle contraction depends on interactions between actin and myosin filaments organized into sarcomeres, but the mechanism by which actin filaments incorporate into sarcomeres remains unclear. We have found that, during larval development in Caenorhabditis elegans, two members of the actin-assembling formin family, CYK-1 and FHOD-1, are present in striated body wall muscles near or on sarcomere Z lines, where barbed ends of actin filaments are anchored. Depletion of either formin during this period stunted growth of the striated contractile lattice, whereas their simultaneous reduction profoundly diminished lattice size and number of striations per muscle cell. CYK-1 persisted at Z lines in adulthood, and its near complete depletion from adults triggered phenotypes ranging from partial loss of Z line-associated filamentous actin to collapse of the contractile lattice. These results are, to our knowledge, the first genetic evidence implicating sarcomere-associated formins in the in vivo organization of the muscle cytoskeleton.
Figures





Similar articles
-
FHOD-1 and profilin protect sarcomeres against contraction-induced deformation> in C. elegans.Mol Biol Cell. 2024 Nov 1;35(11):ar137. doi: 10.1091/mbc.E24-04-0145. Epub 2024 Sep 11. Mol Biol Cell. 2024. PMID: 39259762 Free PMC article.
-
Regulation of structure and function of sarcomeric actin filaments in striated muscle of the nematode Caenorhabditis elegans.Anat Rec (Hoboken). 2014 Sep;297(9):1548-59. doi: 10.1002/ar.22965. Anat Rec (Hoboken). 2014. PMID: 25125169 Free PMC article. Review.
-
FHOD-1 is the only formin in Caenorhabditis elegans that promotes striated muscle growth and Z-line organization in a cell autonomous manner.Cytoskeleton (Hoboken). 2020 Oct;77(10):422-441. doi: 10.1002/cm.21639. Epub 2020 Nov 6. Cytoskeleton (Hoboken). 2020. PMID: 33103378 Free PMC article.
-
FHOD formin and SRF promote post-embryonic striated muscle growth through separate pathways in C. elegans.Exp Cell Res. 2021 Jan 1;398(1):112388. doi: 10.1016/j.yexcr.2020.112388. Epub 2020 Nov 20. Exp Cell Res. 2021. PMID: 33221314 Free PMC article.
-
Dynamic regulation of sarcomeric actin filaments in striated muscle.Cytoskeleton (Hoboken). 2010 Nov;67(11):677-92. doi: 10.1002/cm.20476. Cytoskeleton (Hoboken). 2010. PMID: 20737540 Free PMC article. Review.
Cited by
-
Loss of Sarcomere-associated Formins Disrupts Z-line Organization, but does not Prevent Thin Filament Assembly in Caenorhabditis elegans Muscle.J Cytol Histol. 2015 Mar;6(2):318. doi: 10.4172/2157-7099.1000318. J Cytol Histol. 2015. PMID: 26161293 Free PMC article.
-
The Mechanisms of Thin Filament Assembly and Length Regulation in Muscles.Int J Mol Sci. 2022 May 10;23(10):5306. doi: 10.3390/ijms23105306. Int J Mol Sci. 2022. PMID: 35628117 Free PMC article. Review.
-
Filament-guided filament assembly provides structural memory of filament alignment during cytokinesis.Dev Cell. 2021 Sep 13;56(17):2486-2500.e6. doi: 10.1016/j.devcel.2021.08.009. Epub 2021 Sep 3. Dev Cell. 2021. PMID: 34480876 Free PMC article.
-
FHOD-1 and profilin protect sarcomeres against contraction-induced deformation> in C. elegans.Mol Biol Cell. 2024 Nov 1;35(11):ar137. doi: 10.1091/mbc.E24-04-0145. Epub 2024 Sep 11. Mol Biol Cell. 2024. PMID: 39259762 Free PMC article.
-
CYK-1/Formin activation in cortical RhoA signaling centers promotes organismal left-right symmetry breaking.Proc Natl Acad Sci U S A. 2021 May 18;118(20):e2021814118. doi: 10.1073/pnas.2021814118. Proc Natl Acad Sci U S A. 2021. PMID: 33972425 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

