The versatile molecular complex component LC8 promotes several distinct steps of flagellar assembly
- PMID: 22753897
- PMCID: PMC3392930
- DOI: 10.1083/jcb.201111041
The versatile molecular complex component LC8 promotes several distinct steps of flagellar assembly
Abstract
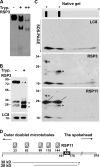
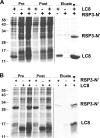
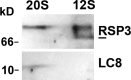
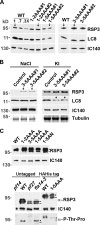
LC8 is present in various molecular complexes. However, its role in these complexes remains unclear. We discovered that although LC8 is a subunit of the radial spoke (RS) complex in Chlamydomonas flagella, it was undetectable in the RS precursor that is converted into the mature RS at the tip of elongating axonemes. Interestingly, LC8 dimers bound in tandem to the N-terminal region of a spoke phosphoprotein, RS protein 3 (RSP3), that docks RSs to axonemes. LC8 enhanced the binding of RSP3 N-terminal fragments to purified axonemes. Likewise, the N-terminal fragments extracted from axonemes contained LC8 and putative spoke-docking proteins. Lastly, perturbations of RSP3's LC8-binding sites resulted in asynchronous flagella with hypophosphorylated RSP3 and defective associations between LC8, RSs, and axonemes. We propose that at the tip of flagella, an array of LC8 dimers binds to RSP3 in RS precursors, triggering phosphorylation, stalk base formation, and axoneme targeting. These multiple effects shed new light on fundamental questions about LC8-containing complexes and axoneme assembly.
Figures





Similar articles
-
In vivo analyses of radial spoke transport, assembly, repair and maintenance.Cytoskeleton (Hoboken). 2018 Aug;75(8):352-362. doi: 10.1002/cm.21457. Epub 2018 Sep 10. Cytoskeleton (Hoboken). 2018. PMID: 30070024 Free PMC article.
-
Assembly of flagellar radial spoke proteins in Chlamydomonas: identification of the axoneme binding domain of radial spoke protein 3.J Cell Biol. 1993 Oct;123(1):183-90. doi: 10.1083/jcb.123.1.183. J Cell Biol. 1993. PMID: 8408197 Free PMC article.
-
The CSC is required for complete radial spoke assembly and wild-type ciliary motility.Mol Biol Cell. 2011 Jul 15;22(14):2520-31. doi: 10.1091/mbc.E11-03-0271. Epub 2011 May 25. Mol Biol Cell. 2011. PMID: 21613541 Free PMC article.
-
Dynamic interactions of dimeric hub proteins underlie their diverse functions and structures: A comparative analysis of 14-3-3 and LC8.J Biol Chem. 2025 Apr;301(4):108416. doi: 10.1016/j.jbc.2025.108416. Epub 2025 Mar 17. J Biol Chem. 2025. PMID: 40107617 Free PMC article. Review.
-
NMR Characterization of Self-Association Domains Promoted by Interactions with LC8 Hub Protein.Comput Struct Biotechnol J. 2014 Feb 25;9:e201402003. doi: 10.5936/csbj.201402003. eCollection 2014. Comput Struct Biotechnol J. 2014. PMID: 24757501 Free PMC article. Review.
Cited by
-
Composition and function of ciliary inner-dynein-arm subunits studied in Chlamydomonas reinhardtii.Cytoskeleton (Hoboken). 2021 Mar;78(3):77-96. doi: 10.1002/cm.21662. Epub 2021 Apr 28. Cytoskeleton (Hoboken). 2021. PMID: 33876572 Free PMC article. Review.
-
The Chlamydomonas mutant pf27 reveals novel features of ciliary radial spoke assembly.Cytoskeleton (Hoboken). 2013 Dec;70(12):804-18. doi: 10.1002/cm.21144. Cytoskeleton (Hoboken). 2013. PMID: 24124175 Free PMC article.
-
Multivalency regulates activity in an intrinsically disordered transcription factor.Elife. 2018 May 1;7:e36258. doi: 10.7554/eLife.36258. Elife. 2018. PMID: 29714690 Free PMC article.
-
The roles of a flagellar HSP40 ensuring rhythmic beating.Mol Biol Cell. 2019 Jan 15;30(2):228-241. doi: 10.1091/mbc.E18-01-0047. Epub 2018 Nov 14. Mol Biol Cell. 2019. PMID: 30427757 Free PMC article.
-
Radial Spokes-A Snapshot of the Motility Regulation, Assembly, and Evolution of Cilia and Flagella.Cold Spring Harb Perspect Biol. 2017 May 1;9(5):a028126. doi: 10.1101/cshperspect.a028126. Cold Spring Harb Perspect Biol. 2017. PMID: 27940518 Free PMC article. Review.
References
-
- Bloch M.A., Johnson K.A.. 1995. Identification of a molecular chaperone in the eukaryotic flagellum and its localization to the site of microtubule assembly. J. Cell Sci. 108:3541–3545. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

