Randomized phase II study of dacomitinib (PF-00299804), an irreversible pan-human epidermal growth factor receptor inhibitor, versus erlotinib in patients with advanced non-small-cell lung cancer
- PMID: 22753918
- PMCID: PMC5321098
- DOI: 10.1200/JCO.2011.40.9433
Randomized phase II study of dacomitinib (PF-00299804), an irreversible pan-human epidermal growth factor receptor inhibitor, versus erlotinib in patients with advanced non-small-cell lung cancer
Abstract
Purpose: This randomized, open-label trial compared dacomitinib (PF-00299804), an irreversible inhibitor of human epidermal growth factor receptors (EGFR)/HER1, HER2, and HER4, with erlotinib, a reversible EGFR inhibitor, in patients with advanced non-small-cell lung cancer (NSCLC).
Patients and methods: Patients with NSCLC, Eastern Cooperative Oncology Group performance status 0 to 2, no prior HER-directed therapy, and one/two prior chemotherapy regimens received dacomitinib 45 mg or erlotinib 150 mg once daily.
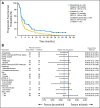
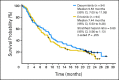
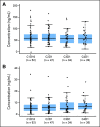
Results: One hundred eighty-eight patients were randomly assigned. Treatment arms were balanced for most clinical and molecular characteristics. Median progression-free survival (PFS; primary end point) was 2.86 months for patients treated with dacomitinib and 1.91 months for patients treated with erlotinib (hazard ratio [HR] = 0.66; 95% CI, 0.47 to 0.91; two-sided P = .012); in patients with KRAS wild-type tumors, median PFS was 3.71 months for patients treated with dacomitinib and 1.91 months for patients treated with erlotinib (HR = 0.55; 95% CI, 0.35 to 0.85; two-sided P = .006); and in patients with KRAS wild-type/EGFR wild-type tumors, median PFS was 2.21 months for patients treated with dacomitinib and 1.84 months for patients treated with erlotinib (HR = 0.61; 95% CI, 0.37 to 0.99; two-sided P = .043). Median overall survival was 9.53 months for patients treated with dacomitinib and 7.44 months for patients treated with erlotinib (HR = 0.80; 95% CI, 0.56 to 1.13; two-sided P = .205). Adverse event-related discontinuations were uncommon in both arms. Common treatment-related adverse events were dermatologic and gastrointestinal, predominantly grade 1 to 2, and more frequent with dacomitinib.
Conclusion: Dacomitinib demonstrated significantly improved PFS versus erlotinib, with acceptable toxicity. PFS benefit was observed in most clinical and molecular subsets, notably KRAS wild-type/EGFR any status, KRAS wild-type/EGFR wild-type, and EGFR mutants.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures



Comment in
-
KRAS wild-type lung cancer: a moving target in an era of genotype migration.J Clin Oncol. 2012 Sep 20;30(27):3322-4. doi: 10.1200/JCO.2012.43.2740. Epub 2012 Aug 6. J Clin Oncol. 2012. PMID: 22869875 No abstract available.
-
To target or not to target, that is the question.J Clin Oncol. 2013 Mar 20;31(9):1254. doi: 10.1200/JCO.2012.45.9818. Epub 2013 Jan 28. J Clin Oncol. 2013. PMID: 23358979 No abstract available.
-
Reply to M.C. Garassino et al.J Clin Oncol. 2013 Mar 20;31(9):1255. doi: 10.1200/JCO.2012.47.7190. J Clin Oncol. 2013. PMID: 23616990 No abstract available.
References
-
- Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. - PubMed
-
- Bell DW, Lynch TJ, Haserlat SM, et al. Epidermal growth factor receptor mutations and gene amplification in non-small-cell lung cancer: Molecular analysis of the IDEAL/INTACT gefitinib trials. J Clin Oncol. 2005;23:8081–8092. - PubMed
-
- Bronte G, Rizzo S, La Paglia L, et al. Driver mutations and differential sensitivity to targeted therapies: A new approach to the treatment of lung adenocarcinoma. Cancer Treat Rev. 2010;36(suppl 3):S21–S29. - PubMed
-
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. - PubMed
-
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): A multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–742. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

