The origins, function, and regulation of T follicular helper cells
- PMID: 22753927
- PMCID: PMC3405510
- DOI: 10.1084/jem.20120994
The origins, function, and regulation of T follicular helper cells
Abstract
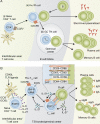
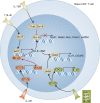
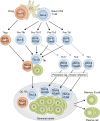
The generation of high-affinity antibodies (Abs) plays a critical role in the neutralization and clearance of pathogens and subsequent host survival after natural infection with a variety of microorganisms. Most currently available vaccines rely on the induction of long-lived protective humoral immune responses by memory B cells and plasma cells, underscoring the importance of Abs in host protection. Ab responses against most antigens (Ags) require interactions between B cells and CD4(+) T helper cells, and it is now well recognized that T follicular helper cells (Tfh) specialize in providing cognate help to B cells and are fundamentally required for the generation of T cell-dependent B cell responses. Perturbations in the development and/or function of Tfh cells can manifest as immunopathologies, such as immunodeficiency, autoimmunity, and malignancy. Unraveling the cellular and molecular requirements underlying Tfh cell formation and maintenance will help to identify molecules that could be targeted for the treatment of immunological diseases that are characterized by insufficient or excessive Ab responses.
Figures
References
-
- Akiba H., Takeda K., Kojima Y., Usui Y., Harada N., Yamazaki T., Ma J., Tezuka K., Yagita H., Okumura K. 2005. The role of ICOS in the CXCR5+ follicular B helper T cell maintenance in vivo. J. Immunol. 175:2340–2348 - PubMed
-
- Al-Herz W., Bousfiha A., Casanova J.L., Chapel H., Conley M.E., Cunningham-Rundles C., Etzioni A., Fischer A., Franco J.L., Geha R.S., et al. 2011. Primary immunodeficiency diseases: an update on the classification from the IUIS Committee. Front. Immun. 2:54 10.3389/fimmu.2011.00054 - DOI - PMC - PubMed
-
- Avery D.T., Bryant V.L., Ma C.S., de Waal Malefyt R., Tangye S.G. 2008. IL-21-induced isotype switching to IgG and IgA by human naive B cells is differentially regulated by IL-4. J. Immunol. 181:1767–1779 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

