ATP release and autocrine signaling through P2X4 receptors regulate γδ T cell activation
- PMID: 22753954
- PMCID: PMC3441317
- DOI: 10.1189/jlb.0312121
ATP release and autocrine signaling through P2X4 receptors regulate γδ T cell activation
Abstract
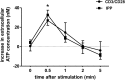
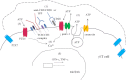
Purinergic signaling plays a key role in a variety of physiological functions, including regulation of immune responses. Conventional αβ T cells release ATP upon TCR cross-linking; ATP binds to purinergic receptors expressed by these cells and triggers T cell activation in an autocrine and paracrine manner. Here, we studied whether similar purinergic signaling pathways also operate in the "unconventional" γδ T lymphocytes. We observed that γδ T cells purified from peripheral human blood rapidly release ATP upon in vitro stimulation with anti-CD3/CD28-coated beads or IPP. Pretreatment of γδ T cells with (10)panx-1, CBX, or Bf A reversed the stimulation-induced increase in extracellular ATP concentration, indicating that panx-1, connexin hemichannels, and vesicular exocytosis contribute to the controlled release of cellular ATP. Blockade of ATP release with (10)panx-1 inhibited Ca(2+) signaling in response to TCR stimulation. qPCR revealed that γδ T cells predominantly express purinergic receptor subtypes A2a, P2X1, P2X4, P2X7, and P2Y11. We found that pharmacological inhibition of P2X4 receptors with TNP-ATP inhibited transcriptional up-regulation of TNF-α and IFN-γ in γδ T cells stimulated with anti-CD3/CD28-coated beads or IPP. Our data thus indicate that purinergic signaling via P2X4 receptors plays an important role in orchestrating the functional response of circulating human γδ T cells.
Figures




Similar articles
-
Pannexin-1 hemichannel-mediated ATP release together with P2X1 and P2X4 receptors regulate T-cell activation at the immune synapse.Blood. 2010 Nov 4;116(18):3475-84. doi: 10.1182/blood-2010-04-277707. Epub 2010 Jul 21. Blood. 2010. PMID: 20660288 Free PMC article.
-
Purinergic P2X7 receptor drives T cell lineage choice and shapes peripheral γδ cells.J Immunol. 2012 Jul 1;189(1):174-80. doi: 10.4049/jimmunol.1101582. Epub 2012 May 30. J Immunol. 2012. PMID: 22649196
-
Hypertonic stress regulates T cell function via pannexin-1 hemichannels and P2X receptors.J Leukoc Biol. 2010 Dec;88(6):1181-9. doi: 10.1189/jlb.0410211. Epub 2010 Sep 30. J Leukoc Biol. 2010. PMID: 20884646 Free PMC article.
-
Structural and Functional Features of the P2X4 Receptor: An Immunological Perspective.Front Immunol. 2021 Mar 25;12:645834. doi: 10.3389/fimmu.2021.645834. eCollection 2021. Front Immunol. 2021. PMID: 33897694 Free PMC article. Review.
-
Mitochondria Synergize With P2 Receptors to Regulate Human T Cell Function.Front Immunol. 2020 Sep 29;11:549889. doi: 10.3389/fimmu.2020.549889. eCollection 2020. Front Immunol. 2020. PMID: 33133068 Free PMC article. Review.
Cited by
-
Purinergic Signaling and the Immune Response in Sepsis: A Review.Clin Ther. 2016 May;38(5):1054-65. doi: 10.1016/j.clinthera.2016.04.002. Epub 2016 May 5. Clin Ther. 2016. PMID: 27156007 Free PMC article. Review.
-
High level expression of A2ARs is required for the enhancing function, but not for the inhibiting function, of γδ T cells in the autoimmune responses of EAU.PLoS One. 2018 Jun 21;13(6):e0199601. doi: 10.1371/journal.pone.0199601. eCollection 2018. PLoS One. 2018. PMID: 29928041 Free PMC article.
-
ATPe Dynamics in Protozoan Parasites. Adapt or Perish.Genes (Basel). 2018 Dec 27;10(1):16. doi: 10.3390/genes10010016. Genes (Basel). 2018. PMID: 30591699 Free PMC article. Review.
-
Purinergic signalling links mechanical breath profile and alveolar mechanics with the pro-inflammatory innate immune response causing ventilation-induced lung injury.Purinergic Signal. 2017 Sep;13(3):363-386. doi: 10.1007/s11302-017-9564-5. Epub 2017 May 26. Purinergic Signal. 2017. PMID: 28547381 Free PMC article. Review.
-
Released ATP Mediates Spermatozoa Chemotaxis Promoted by Uterus-Derived Factor (UDF) in Ascaris suum.Int J Mol Sci. 2022 Apr 6;23(7):4069. doi: 10.3390/ijms23074069. Int J Mol Sci. 2022. PMID: 35409429 Free PMC article.
References
-
- Bonneville M., O'Brien R. L., Born W. K. (2010) γδ T cell effector functions: a blend of innate programming and acquired plasticity. Nat. Rev. Immunol. 10, 467–478 - PubMed
-
- Carding S. R., Egan P. J. (2002) γδ T cells: functional plasticity and heterogeneity. Nat. Rev. Immunol. 2, 336–345 - PubMed
-
- Moser B., Eberl M. (2007) γδ T cells: novel initiators of adaptive immunity. Immunol. Rev. 215, 89–102 - PubMed
-
- Morita C. T., Jin C., Sarikonda G., Wang H. (2007) Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vγ2Vδ2 T cells: discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunol. Rev. 215, 59–76 - PubMed
-
- Hayday A. C. (2000) γδ cells: a right time and a right place for a conserved third way of protection. Annu. Rev. Immunol. 18, 975–1026 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

