Aβ-40 Y10F increases βfibrils formation but attenuates the neurotoxicity of amyloid-β peptide
- PMID: 22754299
- PMCID: PMC3382774
- DOI: 10.3390/ijms13055324
Aβ-40 Y10F increases βfibrils formation but attenuates the neurotoxicity of amyloid-β peptide
Abstract
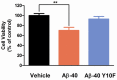
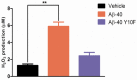
Alzheimer's disease (AD) is characterized by the abnormal aggregation of amyloid-β peptide (Aβ) in extracellular deposits known as senile plaques. The tyrosine residue (Tyr-10) is believed to be important in Aβ-induced neurotoxicity due to the formation of tyrosyl radicals. To reduce the likelihood of cross-linking, here we designed an Aβ-40 analogue (Aβ-40 Y10F) in which the tyrosine residue was substituted by a structurally similar residue, phenylalanine. The aggregation rate was determined by the Thioflavin T (ThT) assay, in which Aβ-40 Y10F populated an ensemble of folded conformations much quicker and stronger than the wild type Aβ. Biophysical tests subsequently confirmed the results of the ThT assay, suggesting the measured increase of β-aggregation may arise predominantly from enhancement of hydrophobicity upon substitution and thus the propensity of intrinsic β-sheet formation. Nevertheless, Aβ-40 Y10F exhibited remarkably decreased neurotoxicity compared to Aβ-40 which could be partly due to the reduced generation of hydrogen peroxide. These findings may lead to further understanding of the structural perturbation of Aβ to its fibrillation.
Keywords: Alzheimer’s disease; amyloid-β peptide; neurotoxicity; βfibrils.
Figures




Similar articles
-
The Effects of N-terminal Mutations on β-amyloid Peptide Aggregation and Toxicity.Neuroscience. 2018 May 21;379:177-188. doi: 10.1016/j.neuroscience.2018.03.014. Epub 2018 Mar 20. Neuroscience. 2018. PMID: 29572166
-
Familial Alzheimer's disease mutations at position 22 of the amyloid β-peptide sequence differentially affect synaptic loss, tau phosphorylation and neuronal cell death in an ex vivo system.PLoS One. 2020 Sep 23;15(9):e0239584. doi: 10.1371/journal.pone.0239584. eCollection 2020. PLoS One. 2020. PMID: 32966331 Free PMC article.
-
Stabilization of native amyloid β-protein oligomers by Copper and Hydrogen peroxide Induced Cross-linking of Unmodified Proteins (CHICUP).Biochim Biophys Acta. 2016 Mar;1864(3):249-259. doi: 10.1016/j.bbapap.2015.12.001. Epub 2015 Dec 15. Biochim Biophys Acta. 2016. PMID: 26699836
-
The Positive Side of the Alzheimer's Disease Amyloid Cross-Interactions: The Case of the Aβ 1-42 Peptide with Tau, TTR, CysC, and ApoA1.Molecules. 2020 May 23;25(10):2439. doi: 10.3390/molecules25102439. Molecules. 2020. PMID: 32456156 Free PMC article. Review.
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
Cited by
-
Chitosan Oligosaccharides Inhibit/Disaggregate Fibrils and Attenuate Amyloid β-Mediated Neurotoxicity.Int J Mol Sci. 2015 May 8;16(5):10526-36. doi: 10.3390/ijms160510526. Int J Mol Sci. 2015. PMID: 26006224 Free PMC article.
-
Key Residue for Aggregation of Amyloid-β Peptides.ACS Chem Neurosci. 2022 Nov 16;13(22):3139-3151. doi: 10.1021/acschemneuro.2c00358. Epub 2022 Oct 27. ACS Chem Neurosci. 2022. PMID: 36302506 Free PMC article.
-
AI-based AlphaFold2 significantly expands the structural space of the autophagy pathway.Autophagy. 2023 Dec;19(12):3201-3220. doi: 10.1080/15548627.2023.2238578. Epub 2023 Jul 30. Autophagy. 2023. PMID: 37516933 Free PMC article.
-
Interleukin-10 Protection against Lipopolysaccharide-Induced Neuro-Inflammation and Neurotoxicity in Ventral Mesencephalic Cultures.Int J Mol Sci. 2015 Dec 28;17(1):25. doi: 10.3390/ijms17010025. Int J Mol Sci. 2015. PMID: 26729090 Free PMC article.
-
Anti-amyloid Aggregation Activity of Natural Compounds: Implications for Alzheimer's Drug Discovery.Mol Neurobiol. 2016 Aug;53(6):3565-3575. doi: 10.1007/s12035-015-9301-4. Epub 2015 Jun 23. Mol Neurobiol. 2016. PMID: 26099310 Review.
References
-
- Selkoe D.J. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–791. - PubMed
-
- Sisodia S.S., Koo E.H., Beyreuther K., Unterbeck A., Price D.L. Evidence that beta-amyloid protein in Alzheimer’s disease is not derived by normal processing. Science. 1990;248:492–495. - PubMed
-
- Seubert P., Vigo-Pelfrey C., Esch F., Lee M., Dovey H., Davis D., Sinha S., Schiossmacher M., Whaley J., Swindlehurst C. Isolation and quantification of soluble Alzheimer’s β-peptide from biological fluids. Nature. 1992;359:325–327. - PubMed
-
- Hardy J., Selkoe D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science. 2002;297:353–356. - PubMed
-
- Dahlgren K.N., Manelli A.M., Stine W.B., Baker L.K., Krafft G.A., LaDu M.J. Oligomeric and fibrillar species of amyloid-β peptides differentially affect neuronal viability. J. Biol. Chem. 2002;277:32046–32053. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

