Role of 14-3-3ζ in platelet glycoprotein Ibα-von Willebrand factor interaction-induced signaling
- PMID: 22754302
- PMCID: PMC3382782
- DOI: 10.3390/ijms13055364
Role of 14-3-3ζ in platelet glycoprotein Ibα-von Willebrand factor interaction-induced signaling
Abstract
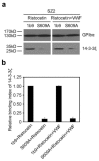
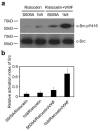
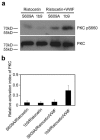
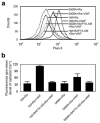
The interaction of platelet glycoprotein (GP) Ib-IX with von Willebrand factor (VWF) exposed at the injured vessel wall or atherosclerotic plaque rupture initiates platelet transient adhesion to the injured vessel wall, which triggers intracellular signaling cascades leading to platelet activation and thrombus formation. 14-3-3ζ has been verified to regulate the VWF binding function of GPIb-IX by interacting with the cytoplasmic domains of GPIb-IX. However, the data regarding the role of 14-3-3ζ in GPIb-IX-VWF interaction-induced signaling still remain controversial. In the present study, the data indicate that the S609A mutation replacing Ser(609) of GPIbα with alanine (S609A) significantly prevented the association of 14-3-3ζ with GPIbα before and after the VWF binding to GPIbα. GPIb-IX-VWF interaction-induced activations of Src family kinases and protein kinase C were clearly reduced in S609A mutation. Furthermore, S609A mutation significantly inhibited GPIb-IX-VWF interaction-induced elevation of cytoplasmic Ca(2+) levels in flow cytometry analysis. Taken together, these data indicate that the association of 14-3-3ζ with the cytoplasmic domain of GPIbα plays an important role in GPIb-IX-VWF interaction-induced signaling.
Keywords: 14-3-3ζ; glycoprotein (GP) Ib-IX; platelets; von Willebrand factor (VWF).
Figures
Similar articles
-
Identification of a novel 14-3-3zeta binding site within the cytoplasmic domain of platelet glycoprotein Ibalpha that plays a key role in regulating the von Willebrand factor binding function of glycoprotein Ib-IX.Circ Res. 2009 Dec 4;105(12):1177-85. doi: 10.1161/CIRCRESAHA.109.204669. Epub 2009 Oct 29. Circ Res. 2009. PMID: 19875727
-
The glycoprotein Ibalpha-von Willebrand factor interaction induces platelet apoptosis.J Thromb Haemost. 2010 Feb;8(2):341-50. doi: 10.1111/j.1538-7836.2009.03653.x. Epub 2009 Oct 19. J Thromb Haemost. 2010. PMID: 19840363
-
Functional association of phosphoinositide-3-kinase with platelet glycoprotein Ibalpha, the major ligand-binding subunit of the glycoprotein Ib-IX-V complex.J Thromb Haemost. 2010 Feb;8(2):324-30. doi: 10.1111/j.1538-7836.2009.03672.x. Epub 2009 Oct 30. J Thromb Haemost. 2010. PMID: 19874472
-
Signaling mechanisms of the platelet glycoprotein Ib-IX complex.Platelets. 2022 Aug 18;33(6):823-832. doi: 10.1080/09537104.2022.2071852. Epub 2022 May 26. Platelets. 2022. PMID: 35615944 Free PMC article. Review.
-
Structural determinants within platelet glycoprotein Ibalpha involved in its binding to von Willebrand factor.Platelets. 2000 Nov;11(7):373-8. doi: 10.1080/09537100020019157. Platelets. 2000. PMID: 11132103 Review.
Cited by
-
14-3-3ζ regulates the mitochondrial respiratory reserve linked to platelet phosphatidylserine exposure and procoagulant function.Nat Commun. 2016 Sep 27;7:12862. doi: 10.1038/ncomms12862. Nat Commun. 2016. PMID: 27670677 Free PMC article.
-
Essential role of glycoprotein Ibα in platelet activation.Blood Adv. 2024 Jul 9;8(13):3388-3401. doi: 10.1182/bloodadvances.2023012308. Blood Adv. 2024. PMID: 38701351 Free PMC article.
References
-
- Lopez J.A. The platelet glycoprotein Ib-IX complex. Blood Coagul. Fibrinolysis. 1994;5:97–119. - PubMed
-
- Du X. Signaling and regulation of the glycoprotein Ib-IX-V complex. Curr. Opin. Hematol. 2007;14:262–269. - PubMed
-
- Kasirer-Friede A., Cozzi M.R., Mazzucato M., De Marco L., Ruggeri Z.M., Shattil S.J. Signaling through GP Ib-IX-V activates αIIbβ3 independently of other receptors. Blood. 2004;103:3403–3411. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

