Dextran and polymer polyethylene glycol (PEG) coating reduce both 5 and 30 nm iron oxide nanoparticle cytotoxicity in 2D and 3D cell culture
- PMID: 22754315
- PMCID: PMC3382777
- DOI: 10.3390/ijms13055554
Dextran and polymer polyethylene glycol (PEG) coating reduce both 5 and 30 nm iron oxide nanoparticle cytotoxicity in 2D and 3D cell culture
Abstract
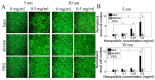
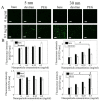
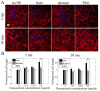
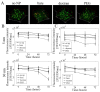
Superparamagnetic iron oxide nanoparticles are widely used in biomedical applications, yet questions remain regarding the effect of nanoparticle size and coating on nanoparticle cytotoxicity. In this study, porcine aortic endothelial cells were exposed to 5 and 30 nm diameter iron oxide nanoparticles coated with either the polysaccharide, dextran, or the polymer polyethylene glycol (PEG). Nanoparticle uptake, cytotoxicity, reactive oxygen species (ROS) formation, and cell morphology changes were measured. Endothelial cells took up nanoparticles of all sizes and coatings in a dose dependent manner, and intracellular nanoparticles remained clustered in cytoplasmic vacuoles. Bare nanoparticles in both sizes induced a more than 6 fold increase in cell death at the highest concentration (0.5 mg/mL) and led to significant cell elongation, whereas cell viability and morphology remained constant with coated nanoparticles. While bare 30 nm nanoparticles induced significant ROS formation, neither 5 nm nanoparticles (bare or coated) nor 30 nm coated nanoparticles changed ROS levels. Furthermore, nanoparticles were more toxic at lower concentrations when cells were cultured within 3D gels. These results indicate that both dextran and PEG coatings reduce nanoparticle cytotoxicity, however different mechanisms may be important for different size nanoparticles.
Keywords: 3D cell culture; PEG; cytotoxicity; dextran; endothelial cells; reactive oxygen species; superparamagnetic iron oxide nanoparticles.
Figures


Similar articles
-
Toxicity of iron oxide nanoparticles: Size and coating effects.J Biochem Mol Toxicol. 2018 Dec;32(12):e22225. doi: 10.1002/jbt.22225. Epub 2018 Oct 5. J Biochem Mol Toxicol. 2018. PMID: 30290022
-
Effects of Iron-Oxide Nanoparticle Surface Chemistry on Uptake Kinetics and Cytotoxicity in CHO-K1 Cells.Int J Mol Sci. 2015 Dec 31;17(1):54. doi: 10.3390/ijms17010054. Int J Mol Sci. 2015. PMID: 26729108 Free PMC article.
-
Superparamagnetic iron oxide nanoparticles change endothelial cell morphology and mechanics via reactive oxygen species formation.J Biomed Mater Res A. 2011 Jan;96(1):186-95. doi: 10.1002/jbm.a.32972. Epub 2010 Nov 9. J Biomed Mater Res A. 2011. PMID: 21105167
-
Assessing cytotoxicity of (iron oxide-based) nanoparticles: an overview of different methods exemplified with cationic magnetoliposomes.Contrast Media Mol Imaging. 2009 Sep-Oct;4(5):207-19. doi: 10.1002/cmmi.282. Contrast Media Mol Imaging. 2009. PMID: 19810053 Review.
-
Recent advances in stealth coating of nanoparticle drug delivery systems.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2012 Mar-Apr;4(2):219-33. doi: 10.1002/wnan.1157. Epub 2012 Jan 9. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2012. PMID: 22231928 Free PMC article. Review.
Cited by
-
Synthesis and functionalization of monodisperse near-ultraviolet and visible excitable multifunctional Eu(3+), Bi(3+):REVO4 nanophosphors for bioimaging and biosensing applications.Nanoscale. 2016 Jun 16;8(24):12221-36. doi: 10.1039/c6nr03369e. Nanoscale. 2016. PMID: 27253384 Free PMC article.
-
Capping Agent-Dependent Toxicity and Antimicrobial Activity of Silver Nanoparticles: An In Vitro Study. Concerns about Potential Application in Dental Practice.Int J Med Sci. 2016 Sep 27;13(10):772-782. doi: 10.7150/ijms.16011. eCollection 2016. Int J Med Sci. 2016. PMID: 27766027 Free PMC article.
-
Targeted Superparamagnetic Iron Oxide Nanoparticles for In Vivo Magnetic Resonance Imaging of T-Cells in Rheumatoid Arthritis.Mol Imaging Biol. 2017 Apr;19(2):233-244. doi: 10.1007/s11307-016-1001-6. Mol Imaging Biol. 2017. PMID: 27572293
-
Layered double hydroxide nanocomposite for drug delivery systems; bio-distribution, toxicity and drug activity enhancement.Chem Cent J. 2014 Aug 10;8(1):47. doi: 10.1186/s13065-014-0047-2. eCollection 2014. Chem Cent J. 2014. PMID: 25177361 Free PMC article. Review.
-
Fabrication of anionic dextran-coated micelles for aptamer targeted delivery of camptothecin and survivin-shRNA to colon adenocarcinoma.Gene Ther. 2022 Feb;29(1-2):55-68. doi: 10.1038/s41434-021-00234-0. Epub 2021 Feb 25. Gene Ther. 2022. PMID: 33633357
References
-
- Pouliquen D., Le Jeune J.J., Perdrisot R., Ermias A., Jallet P. Iron oxide nanoparticles for use as an MRI contrast agent: Pharmacokinetics and metabolism. Magn. Reson. Imag. 1991;9:275–283. - PubMed
-
- Fortin J.-P., Wilhelm C., Servais J., Ménager C., Bacri J.-C., Gazeau F. Size-sorted anionic iron oxide nanomagnets as colloidal mediators for magnetic hyperthermia. J. Am. Chem. Soc. 2007;129:2628–2635. - PubMed
-
- Babič M., Horák D., Trchová M., Jendelová P., Glogarová K.I., Lesný P., Herynek V., Hájek M., Syková E. Poly(l-lysine)-modified iron oxide nanoparticles for stem cell labeling. Bioconjugate Chem. 2008;19:740–750. - PubMed
-
- Tartaj P., Morales M.D.P., Veintemillas-Verdaguer S., González-Carreño T., Serna C.J. The preparation of magnetic nanoparticles for applications in biomedicine. J. Phys. Appl. Phys. 2003;36:R182–R197.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

