Oculomotor learning revisited: a model of reinforcement learning in the basal ganglia incorporating an efference copy of motor actions
- PMID: 22754501
- PMCID: PMC3385561
- DOI: 10.3389/fncir.2012.00038
Oculomotor learning revisited: a model of reinforcement learning in the basal ganglia incorporating an efference copy of motor actions
Abstract
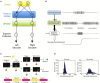
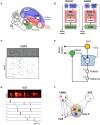
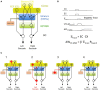
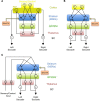
In its simplest formulation, reinforcement learning is based on the idea that if an action taken in a particular context is followed by a favorable outcome, then, in the same context, the tendency to produce that action should be strengthened, or reinforced. While reinforcement learning forms the basis of many current theories of basal ganglia (BG) function, these models do not incorporate distinct computational roles for signals that convey context, and those that convey what action an animal takes. Recent experiments in the songbird suggest that vocal-related BG circuitry receives two functionally distinct excitatory inputs. One input is from a cortical region that carries context information about the current "time" in the motor sequence. The other is an efference copy of motor commands from a separate cortical brain region that generates vocal variability during learning. Based on these findings, I propose here a general model of vertebrate BG function that combines context information with a distinct motor efference copy signal. The signals are integrated by a learning rule in which efference copy inputs gate the potentiation of context inputs (but not efference copy inputs) onto medium spiny neurons in response to a rewarded action. The hypothesis is described in terms of a circuit that implements the learning of visually guided saccades. The model makes testable predictions about the anatomical and functional properties of hypothesized context and efference copy inputs to the striatum from both thalamic and cortical sources.
Keywords: context; corticostriatal; efference copy; motor learning; songbird; striatum; thalamostriatal.
Figures
Similar articles
-
What Is the Role of Thalamostriatal Circuits in Learning Vocal Sequences?Front Neural Circuits. 2021 Sep 22;15:724858. doi: 10.3389/fncir.2021.724858. eCollection 2021. Front Neural Circuits. 2021. PMID: 34630047 Free PMC article.
-
The role of efference copy in striatal learning.Curr Opin Neurobiol. 2014 Apr;25:194-200. doi: 10.1016/j.conb.2014.01.012. Epub 2014 Feb 21. Curr Opin Neurobiol. 2014. PMID: 24566242 Free PMC article. Review.
-
The Avian Basal Ganglia Are a Source of Rapid Behavioral Variation That Enables Vocal Motor Exploration.J Neurosci. 2018 Nov 7;38(45):9635-9647. doi: 10.1523/JNEUROSCI.2915-17.2018. Epub 2018 Sep 24. J Neurosci. 2018. PMID: 30249800 Free PMC article.
-
An associational model of birdsong sensorimotor learning I. Efference copy and the learning of song syllables.J Neurophysiol. 2000 Sep;84(3):1204-23. doi: 10.1152/jn.2000.84.3.1204. J Neurophysiol. 2000. PMID: 10979996
-
Variability in action: Contributions of a songbird cortical-basal ganglia circuit to vocal motor learning and control.Neuroscience. 2015 Jun 18;296:39-47. doi: 10.1016/j.neuroscience.2014.10.010. Epub 2014 Oct 18. Neuroscience. 2015. PMID: 25445191 Review.
Cited by
-
Familial bias and auditory feedback regulation of vocal babbling patterns during early song development.Sci Rep. 2016 Jul 22;6:30323. doi: 10.1038/srep30323. Sci Rep. 2016. PMID: 27444993 Free PMC article.
-
BOLD differences normally attributed to inhibitory control predict symptoms, not task-directed inhibitory control in ADHD.J Neurodev Disord. 2020 Feb 21;12(1):8. doi: 10.1186/s11689-020-09311-8. J Neurodev Disord. 2020. PMID: 32085698 Free PMC article.
-
Investigation of motor self-monitoring deficits in schizophrenia with passivity experiences using a novel modified joint position matching paradigm.Eur Arch Psychiatry Clin Neurosci. 2022 Apr;272(3):509-518. doi: 10.1007/s00406-021-01261-z. Epub 2021 Apr 10. Eur Arch Psychiatry Clin Neurosci. 2022. PMID: 33837844
-
Birds, primates, and spoken language origins: behavioral phenotypes and neurobiological substrates.Front Evol Neurosci. 2012 Aug 16;4:12. doi: 10.3389/fnevo.2012.00012. eCollection 2012. Front Evol Neurosci. 2012. PMID: 22912615 Free PMC article.
-
Developmentally regulated pathways for motor skill learning in songbirds.J Comp Neurol. 2022 Jun;530(8):1288-1301. doi: 10.1002/cne.25276. Epub 2021 Dec 14. J Comp Neurol. 2022. PMID: 34818442 Free PMC article.
References
-
- Alexander G. E., Crutcher M. D. (1990). Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 13, 266–271 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

