The selective neurotoxin DSP-4 impairs the noradrenergic projections from the locus coeruleus to the inferior colliculus in rats
- PMID: 22754504
- PMCID: PMC3385004
- DOI: 10.3389/fncir.2012.00041
The selective neurotoxin DSP-4 impairs the noradrenergic projections from the locus coeruleus to the inferior colliculus in rats
Abstract
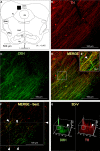
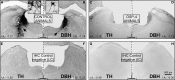
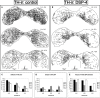
The inferior colliculus (IC) and the locus coeruleus (LC) are two midbrain nuclei that integrate multimodal information and play a major role in novelty detection to elicit an orienting response. Despite the reciprocal connections between these two structures, the projection pattern and target areas of the LC within the subdivisions of the rat IC are still unknown. Here, we used tract-tracing approaches combined with immunohistochemistry, densitometry, and confocal microscopy (CM) analysis to describe a projection from the LC to the IC. Biotinylated dextran amine (BDA) injections into the LC showed that the LC-IC projection is mainly ipsilateral (90%) and reaches, to a major extent, the dorsal and lateral part of the IC and the intercollicular commissure. Additionally, some LC fibers extend into the central nucleus of the IC. The neurochemical nature of this projection is noradrenergic, given that tyrosine hydroxylase (TH) and dopamine beta hydroxylase (DBH) colocalize with the BDA-labeled fibers from the LC. To determine the total field of the LC innervations in the IC, we destroyed the LC neurons and fibers using a highly selective neurotoxin, DSP-4, and then studied the distribution and density of TH- and DBH-immunolabeled axons in the IC. In the DSP-4 treated animals, the number of axonal fibers immunolabeled for TH and DBH were deeply decreased throughout the entire rostrocaudal extent of the IC and its subdivisions compared to controls. Our densitometry results showed that the IC receives up to 97% of its noradrenergic innervations from the LC neurons and only 3% from non-coeruleus neurons. Our results also indicate that TH immunoreactivity in the IC was less impaired than the immunoreactivity for DBH after DSP-4 administration. This is consistent with the existence of an important dopaminergic projection from the substantia nigra to the IC. In conclusion, our study demonstrates and quantifies the noradrenergic projection from the LC to the IC and its subdivisions. The re-examination of the TH and DBH immunoreactivity after DSP-4 treatment provides insights into the source, extent, and topographic distribution of the LC efferent network in the IC, and hence, contributes to our understanding of the role of the noradrenaline (NA) system in auditory processing.
Keywords: biotinylated dextran amine; densitometry; dopamine beta hydroxylase deficiency; immunohistochemistry; neurotoxicity; noradrenergic efferents; noradrenergic modulation; tyrosine hydroxylase.
Figures




Similar articles
-
Distribution and origin of noradrenergic and serotonergic fibers in the cochlear nucleus and inferior colliculus of the rat.Brain Res. 1991 Aug 23;557(1-2):190-201. doi: 10.1016/0006-8993(91)90134-h. Brain Res. 1991. PMID: 1747753
-
Dopamine in Auditory Nuclei and Lemniscal Projections is Poised to Influence Acoustic Integration in the Inferior Colliculus.Front Neural Circuits. 2021 Mar 5;15:624563. doi: 10.3389/fncir.2021.624563. eCollection 2021. Front Neural Circuits. 2021. PMID: 33746717 Free PMC article.
-
Input from central nucleus of the amygdala efferents to pericoerulear dendrites, some of which contain tyrosine hydroxylase immunoreactivity.J Neurosci Res. 1996 Aug 1;45(3):289-302. doi: 10.1002/(SICI)1097-4547(19960801)45:3<289::AID-JNR11>3.0.CO;2-#. J Neurosci Res. 1996. PMID: 8841990
-
Selective effects of DSP-4 on locus coeruleus axons: are there pharmacologically different types of noradrenergic axons in the central nervous system?Prog Brain Res. 1991;88:257-68. doi: 10.1016/s0079-6123(08)63815-7. Prog Brain Res. 1991. PMID: 1726027 Review.
-
Causes, consequences, and cures for neuroinflammation mediated via the locus coeruleus: noradrenergic signaling system.J Neurochem. 2016 Oct;139 Suppl 2:154-178. doi: 10.1111/jnc.13447. Epub 2016 Mar 10. J Neurochem. 2016. PMID: 26968403 Review.
Cited by
-
The Inferior Colliculus in Alcoholism and Beyond.Front Syst Neurosci. 2020 Dec 11;14:606345. doi: 10.3389/fnsys.2020.606345. eCollection 2020. Front Syst Neurosci. 2020. PMID: 33362482 Free PMC article. Review.
-
Dopamine in the auditory brainstem and midbrain: co-localization with amino acid neurotransmitters and gene expression following cochlear trauma.Front Neuroanat. 2015 Jul 22;9:88. doi: 10.3389/fnana.2015.00088. eCollection 2015. Front Neuroanat. 2015. PMID: 26257610 Free PMC article.
-
Foreground stimuli and task engagement enhance neuronal adaptation to background noise in the inferior colliculus of macaques.J Neurophysiol. 2020 Nov 1;124(5):1315-1326. doi: 10.1152/jn.00153.2020. Epub 2020 Sep 16. J Neurophysiol. 2020. PMID: 32937088 Free PMC article.
-
Noninvasive Bimodal Neuromodulation for the Treatment of Tinnitus: Protocol for a Second Large-Scale Double-Blind Randomized Clinical Trial to Optimize Stimulation Parameters.JMIR Res Protoc. 2019 Sep 27;8(9):e13176. doi: 10.2196/13176. JMIR Res Protoc. 2019. PMID: 31573942 Free PMC article.
-
Relationships between Pupil Diameter and Neuronal Activity in the Locus Coeruleus, Colliculi, and Cingulate Cortex.Neuron. 2016 Jan 6;89(1):221-34. doi: 10.1016/j.neuron.2015.11.028. Epub 2015 Dec 17. Neuron. 2016. PMID: 26711118 Free PMC article.
References
-
- Aston-Jones G. (2004). “Locus coeruleus, A5 and A7 noradrenergic cell groups,” in The Rat Nervous System, ed Paxinos G. (San Diego, CA: Elsevier; ), 259–294
-
- Aston-Jones G., Chiang C., Alexinsky T. (1991). Discharge of noradrenergic locus coeruleus neurons in behaving rats and monkeys suggests a role in vigilance. Prog. Brain Res. 88, 501–520 - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

