Three-dimensional eye position signals shape both peripersonal space and arm movement activity in the medial posterior parietal cortex
- PMID: 22754511
- PMCID: PMC3385520
- DOI: 10.3389/fnint.2012.00037
Three-dimensional eye position signals shape both peripersonal space and arm movement activity in the medial posterior parietal cortex
Abstract
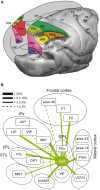
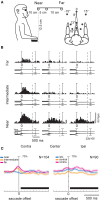
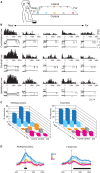
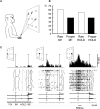
Research conducted over the last decades has established that the medial part of posterior parietal cortex (PPC) is crucial for controlling visually guided actions in human and non-human primates. Within this cortical sector there is area V6A, a crucial node of the parietofrontal network involved in arm movement control in both monkeys and humans. However, the encoding of action-in-depth by V6A cells had been not studied till recently. Recent neurophysiological studies show the existence in V6A neurons of signals related to the distance of targets from the eyes. These signals are integrated, often at the level of single cells, with information about the direction of gaze, thus encoding spatial location in 3D space. Moreover, 3D eye position signals seem to be further exploited at two additional levels of neural processing: (a) in determining whether targets are located in the peripersonal space or not, and (b) in shaping the spatial tuning of arm movement related activity toward reachable targets. These findings are in line with studies in putative homolog regions in humans and together point to a role of medial PPC in encoding both the vergence angle of the eyes and peripersonal space. Besides its role in spatial encoding also in depth, several findings demonstrate the involvement of this cortical sector in non-spatial processes.
Keywords: eye-hand coordination; fixation depth; gaze; reaching; sensorimotor transformation; vergence; version.
Figures

Similar articles
-
Fix your eyes in the space you could reach: neurons in the macaque medial parietal cortex prefer gaze positions in peripersonal space.PLoS One. 2011;6(8):e23335. doi: 10.1371/journal.pone.0023335. Epub 2011 Aug 17. PLoS One. 2011. PMID: 21858075 Free PMC article.
-
Eye position encoding in three-dimensional space: integration of version and vergence signals in the medial posterior parietal cortex.J Neurosci. 2012 Jan 4;32(1):159-69. doi: 10.1523/JNEUROSCI.4028-11.2012. J Neurosci. 2012. PMID: 22219279 Free PMC article.
-
Common neural substrate for processing depth and direction signals for reaching in the monkey medial posterior parietal cortex.Cereb Cortex. 2014 Jun;24(6):1645-57. doi: 10.1093/cercor/bht021. Epub 2013 Feb 4. Cereb Cortex. 2014. PMID: 23382514
-
Parieto-frontal coding of reaching: an integrated framework.Exp Brain Res. 1999 Dec;129(3):325-46. doi: 10.1007/s002210050902. Exp Brain Res. 1999. PMID: 10591906 Review.
-
Vision for Prehension in the Medial Parietal Cortex.Cereb Cortex. 2017 Feb 1;27(2):1149-1163. doi: 10.1093/cercor/bhv302. Cereb Cortex. 2017. PMID: 26656999 Review.
Cited by
-
Selectivity to translational egomotion in human brain motion areas.PLoS One. 2013;8(4):e60241. doi: 10.1371/journal.pone.0060241. Epub 2013 Apr 5. PLoS One. 2013. PMID: 23577096 Free PMC article.
-
Functional Neuroanatomy of the Human Accommodation Response to an "E" Target Varying from -3 to -6 Diopters.Front Integr Neurosci. 2020 May 21;14:29. doi: 10.3389/fnint.2020.00029. eCollection 2020. Front Integr Neurosci. 2020. PMID: 32508603 Free PMC article.
-
Encoding of movement in near extrapersonal space in primate area VIP.Front Behav Neurosci. 2013 Feb 13;7:8. doi: 10.3389/fnbeh.2013.00008. eCollection 2013. Front Behav Neurosci. 2013. PMID: 23407621 Free PMC article.
-
Cerebral complexity preceded enlarged brain size and reduced olfactory bulbs in Old World monkeys.Nat Commun. 2015 Jul 3;6:7580. doi: 10.1038/ncomms8580. Nat Commun. 2015. PMID: 26138795 Free PMC article.
-
Cortical Afferents and Myeloarchitecture Distinguish the Medial Intraparietal Area (MIP) from Neighboring Subdivisions of the Macaque Cortex.eNeuro. 2017 Dec 8;4(6):ENEURO.0344-17.2017. doi: 10.1523/ENEURO.0344-17.2017. eCollection 2017 Nov-Dec. eNeuro. 2017. PMID: 29379868 Free PMC article.
References
LinkOut - more resources
Full Text Sources

