Persistence and storage of activity patterns in spiking recurrent cortical networks: modulation of sigmoid signals by after-hyperpolarization currents and acetylcholine
- PMID: 22754524
- PMCID: PMC3386521
- DOI: 10.3389/fncom.2012.00042
Persistence and storage of activity patterns in spiking recurrent cortical networks: modulation of sigmoid signals by after-hyperpolarization currents and acetylcholine
Abstract
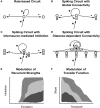
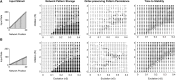
Many cortical networks contain recurrent architectures that transform input patterns before storing them in short-term memory (STM). Theorems in the 1970's showed how feedback signal functions in rate-based recurrent on-center off-surround networks control this process. A sigmoid signal function induces a quenching threshold below which inputs are suppressed as noise and above which they are contrast-enhanced before pattern storage. This article describes how changes in feedback signaling, neuromodulation, and recurrent connectivity may alter pattern processing in recurrent on-center off-surround networks of spiking neurons. In spiking neurons, fast, medium, and slow after-hyperpolarization (AHP) currents control sigmoid signal threshold and slope. Modulation of AHP currents by acetylcholine (ACh) can change sigmoid shape and, with it, network dynamics. For example, decreasing signal function threshold and increasing slope can lengthen the persistence of a partially contrast-enhanced pattern, increase the number of active cells stored in STM, or, if connectivity is distance-dependent, cause cell activities to cluster. These results clarify how cholinergic modulation by the basal forebrain may alter the vigilance of category learning circuits, and thus their sensitivity to predictive mismatches, thereby controlling whether learned categories code concrete or abstract features, as predicted by Adaptive Resonance Theory. The analysis includes global, distance-dependent, and interneuron-mediated circuits. With an appropriate degree of recurrent excitation and inhibition, spiking networks maintain a partially contrast-enhanced pattern for 800 ms or longer after stimuli offset, then resolve to no stored pattern, or to winner-take-all (WTA) stored patterns with one or multiple winners. Strengthening inhibition prolongs a partially contrast-enhanced pattern by slowing the transition to stability, while strengthening excitation causes more winners when the network stabilizes.
Keywords: Adaptive Resonance Theory; acetylcholine modulation; after-hyperpolarization current; pattern processing; short-term memory; sigmoid signal; spiking neuron; vigilance.
Figures









Similar articles
-
After-hyperpolarization currents and acetylcholine control sigmoid transfer functions in a spiking cortical model.J Comput Neurosci. 2012 Apr;32(2):253-80. doi: 10.1007/s10827-011-0354-8. Epub 2011 Jul 21. J Comput Neurosci. 2012. PMID: 21779754
-
Joining distributed pattern processing and homeostatic plasticity in recurrent on-center off-surround shunting networks: noise, saturation, short-term memory, synaptic scaling, and BDNF.Neural Netw. 2012 Jan;25(1):21-9. doi: 10.1016/j.neunet.2011.07.009. Epub 2011 Aug 12. Neural Netw. 2012. PMID: 21890320
-
Acetylcholine Neuromodulation in Normal and Abnormal Learning and Memory: Vigilance Control in Waking, Sleep, Autism, Amnesia and Alzheimer's Disease.Front Neural Circuits. 2017 Nov 2;11:82. doi: 10.3389/fncir.2017.00082. eCollection 2017. Front Neural Circuits. 2017. PMID: 29163063 Free PMC article. Review.
-
Versatile networks of simulated spiking neurons displaying winner-take-all behavior.Front Comput Neurosci. 2013 Mar 19;7:16. doi: 10.3389/fncom.2013.00016. eCollection 2013. Front Comput Neurosci. 2013. PMID: 23515493 Free PMC article.
-
Unraveling the attentional functions of cortical cholinergic inputs: interactions between signal-driven and cognitive modulation of signal detection.Brain Res Brain Res Rev. 2005 Feb;48(1):98-111. doi: 10.1016/j.brainresrev.2004.08.006. Brain Res Brain Res Rev. 2005. PMID: 15708630 Review.
Cited by
-
The Emotional Gatekeeper: A Computational Model of Attentional Selection and Suppression through the Pathway from the Amygdala to the Inhibitory Thalamic Reticular Nucleus.PLoS Comput Biol. 2016 Feb 1;12(2):e1004722. doi: 10.1371/journal.pcbi.1004722. eCollection 2016 Feb. PLoS Comput Biol. 2016. PMID: 26828203 Free PMC article.
-
Neural Dynamics of Autistic Repetitive Behaviors and Fragile X Syndrome: Basal Ganglia Movement Gating and mGluR-Modulated Adaptively Timed Learning.Front Psychol. 2018 Mar 13;9:269. doi: 10.3389/fpsyg.2018.00269. eCollection 2018. Front Psychol. 2018. PMID: 29593596 Free PMC article.
-
How reduction of theta rhythm by medial septum inactivation may covary with disruption of entorhinal grid cell responses due to reduced cholinergic transmission.Front Neural Circuits. 2013 Oct 31;7:173. doi: 10.3389/fncir.2013.00173. eCollection 2013. Front Neural Circuits. 2013. PMID: 24198762 Free PMC article.
-
A Neural Model of Intrinsic and Extrinsic Hippocampal Theta Rhythms: Anatomy, Neurophysiology, and Function.Front Syst Neurosci. 2021 Apr 28;15:665052. doi: 10.3389/fnsys.2021.665052. eCollection 2021. Front Syst Neurosci. 2021. PMID: 33994965 Free PMC article. Review.
-
Editorial: State-dependent brain computation.Front Comput Neurosci. 2015 Jun 23;9:77. doi: 10.3389/fncom.2015.00077. eCollection 2015. Front Comput Neurosci. 2015. PMID: 26157384 Free PMC article. No abstract available.
References
-
- Arnold H. M., Burk J. A., Hodgson E. M., Sarter M., Bruno J. P. (2002). Differential cortical acetylcholine release in rats performing a sustained attention task versus behavioral control tasks that do not explicitly tax attention. Neuroscience 114, 451–460 10.1016/S0306-4522(02)00292-0 - DOI - PubMed
-
- Atri A., Sherman S., Norman K. A., Kirchhoff B. A., Nicolas M. M., Greicius M. D., Cramer S. C., Breiter H. C., Hasselmo M. E., Stern C. E. (2004). Blockade of central cholinergic receptors impairs new learning and increases proactive interference in a word paired-associate memory task. Behav. Neurosci. 118, 223–236 10.1037/0735-7044.118.1.223 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

