Arrhythmogenic right ventricular cardiomyopathy: considerations from in silico experiments
- PMID: 22754532
- PMCID: PMC3385583
- DOI: 10.3389/fphys.2012.00168
Arrhythmogenic right ventricular cardiomyopathy: considerations from in silico experiments
Abstract
Objective: Arrhythmogenic right ventricular cardiomyopathy (ARVC) is associated with remodeling of gap junctions and also, although less well-defined, down-regulation of the fast sodium current. The gap junction remodeling and down-regulation of sodium current have been proposed as contributors to arrhythmogenesis in ARVC by slowing conduction. The objective of the present study was to assess the amount of conduction slowing due to the observed gap junction remodeling and down-regulation of sodium current.
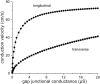
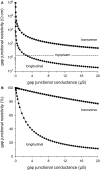
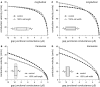
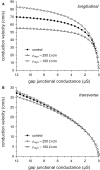
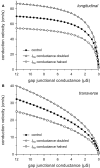
Methods: The effects of (changes in) gap junctional conductance, cell dimensions, and sodium current on both longitudinal and transversal conduction velocity were tested by simulating action potential propagation in linear strands of human ventricular cells that were either arranged end-to-end or side-by-side.
Results: A 50% reduction in gap junction content, as commonly observed in ARVC, gives rise to an 11% decrease in longitudinal conduction velocity and a 29% decrease in transverse conduction velocity. A down-regulation of the sodium current through a 50% decrease in peak current density as well as a -15 mV shift in steady-state inactivation, as observed in an experimental model of ARVC, decreases conduction velocity in either direction by 32%. In combination, the gap junction remodeling and down-regulation of sodium current result in a 40% decrease in longitudinal conduction velocity and a 52% decrease in transverse conduction velocity.
Conclusion: The gap junction remodeling and down-regulation of sodium current do result in conduction slowing, but heterogeneity of gap junction remodeling, in combination with down-regulation of sodium current, rather than gap junction remodeling per se may be a critical factor in arrhythmogenesis in ARVC.
Keywords: arrhythmogenic right ventricular cardiomyopathy; cardiac arrhythmias; cardiac electrophysiology; cardiac myocytes; computer simulations; connexin43; gap junctions; sodium channels.
Figures



References
-
- Basso C., Czarnowska E., Della Barbera M., Bauce B., Beffagna G., Wlodarska E. K., Pilichou K., Ramondo A., Lorenzon A., Wozniek O., Corrado D., Daliento L., Danieli G. A., Valente M., Nava A., Thiene G., Rampazzo A. (2006). Ultrastructural evidence of intercalated disc remodelling in arrhythmogenic right ventricular cardiomyopathy: an electron microscopy investigation on endomyocardial biopsies. Eur. Heart J. 27, 1847–1854 10.1093/eurheartj/ehl095 - DOI - PubMed
-
- Basso C., Fox P. R., Meurs K. M., Towbin J. A., Spier A. W., Calabrese F., Maron B. J., Thiene G. (2004). Arrhythmogenic right ventricular cardiomyopathy causing sudden cardiac death in Boxer dogs: a new animal model of human disease. Circulation 109, 1180–1185 10.1161/01.CIR.0000118494.07530.65 - DOI - PubMed
-
- Bhuiyan Z. A., Jongbloed J. D. H., van der Smagt J., Lombardi P. M., Wiesfeld A. C. P., Nelen M., Schouten M., Jongbloed R., Cox M. G. P. J., van Wolferen M., Rodriguez L. M., van Gelder I. C., Bikker H., Suurmeijer A. J. H., van den Berg M. P., Mannens M. M. A. M., Hauer R. N. W., Wilde A. A. M., van Tintelen J. P. (2009). Desmoglein-2 and desmocollin-2 mutations in Dutch arrhythmogenic right ventricular dysplasia/cardiomypathy patients: results from a multicenter study. Circ. Cardiovasc. Genet. 2, 418–427 10.1161/CIRCGENETICS.108.839829 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

