Discharge Identity of Medullary Inspiratory Neurons is Altered during Repetitive Fictive Cough
- PMID: 22754536
- PMCID: PMC3386566
- DOI: 10.3389/fphys.2012.00223
Discharge Identity of Medullary Inspiratory Neurons is Altered during Repetitive Fictive Cough
Abstract
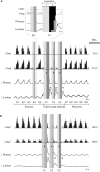
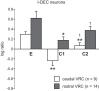
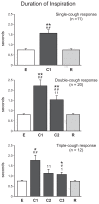
This study investigated the stability of the discharge identity of inspiratory decrementing (I-Dec) and augmenting (I-Aug) neurons in the caudal (cVRC) and rostral (rVRC) ventral respiratory column during repetitive fictive cough in the cat. Inspiratory neurons in the cVRC (n = 23) and rVRC (n = 17) were recorded with microelectrodes. Fictive cough was elicited by mechanical stimulation of the intrathoracic trachea. Approximately 43% (10 of 23) of I-Dec neurons shifted to an augmenting discharge pattern during the first cough cycle (C1). By the second cough cycle (C2), half of these returned to a decrementing pattern. Approximately 94% (16 of 17) of I-Aug neurons retained an augmenting pattern during C1 of a multi-cough response episode. Phrenic burst amplitude and inspiratory duration increased during C1, but decreased with each subsequent cough in a series of repetitive coughs. As a step in evaluating the model-driven hypothesis that VRC I-Dec neurons contribute to the augmentation of inspiratory drive during cough via inhibition of VRC tonic expiratory neurons that inhibit premotor inspiratory neurons, cross-correlation analysis was used to assess relationships of tonic expiratory cells with simultaneously recorded inspiratory neurons. Our results suggest that reconfiguration of inspiratory-related sub-networks of the respiratory pattern generator occurs on a cycle-by-cycle basis during repetitive coughing.
Keywords: breathing; cough; expiratory; inspiratory; medulla; respiratory pattern generator.
Figures




Similar articles
-
Functional connectivity among ventrolateral medullary respiratory neurones and responses during fictive cough in the cat.J Physiol. 2000 May 15;525 Pt 1(Pt 1):207-24. doi: 10.1111/j.1469-7793.2000.00207.x. J Physiol. 2000. PMID: 10811738 Free PMC article.
-
Activity of bulbar respiratory neurons during fictive coughing and swallowing in the decerebrate cat.J Physiol. 1994 Oct 15;480 ( Pt 2)(Pt 2):309-24. doi: 10.1113/jphysiol.1994.sp020361. J Physiol. 1994. PMID: 7869246 Free PMC article.
-
Medullary respiratory neurones and control of laryngeal motoneurones during fictive eupnoea and cough in the cat.J Physiol. 2001 Jul 15;534(Pt. 2):565-81. doi: 10.1111/j.1469-7793.2001.t01-1-00565.x. J Physiol. 2001. PMID: 11454973 Free PMC article.
-
Midbrain control of breathing and blood pressure: The role of periaqueductal gray matter and mesencephalic collicular neuronal microcircuit oscillators.Eur J Neurosci. 2020 Oct;52(8):3879-3902. doi: 10.1111/ejn.14727. Epub 2020 May 1. Eur J Neurosci. 2020. Retraction in: Eur J Neurosci. 2021 Oct;54(7):6685. doi: 10.1111/ejn.15484. PMID: 32227408 Retracted. Review.
-
Central nervous mechanisms of cough.Pulm Pharmacol Ther. 2002;15(3):227-33. doi: 10.1006/pupt.2002.0358. Pulm Pharmacol Ther. 2002. PMID: 12099769 Review.
Cited by
-
Inputs to medullary respiratory neurons from a pontine subregion that controls breathing frequency.Respir Physiol Neurobiol. 2019 Jul;265:127-140. doi: 10.1016/j.resp.2018.06.011. Epub 2018 Jun 28. Respir Physiol Neurobiol. 2019. PMID: 29964165 Free PMC article.
-
Blood pressure drives multispectral tuning of inspiration via a linked-loop neural network.J Neurophysiol. 2020 Dec 1;124(6):1676-1697. doi: 10.1152/jn.00442.2020. Epub 2020 Sep 23. J Neurophysiol. 2020. PMID: 32965158 Free PMC article.
-
Carotid Bodies and the Integrated Cardiorespiratory Response to Hypoxia.Physiology (Bethesda). 2018 Jul 1;33(4):281-297. doi: 10.1152/physiol.00014.2018. Physiology (Bethesda). 2018. PMID: 29897299 Free PMC article. Review.
-
Peripheral chemoreceptors tune inspiratory drive via tonic expiratory neuron hubs in the medullary ventral respiratory column network.J Neurophysiol. 2015 Jan 1;113(1):352-68. doi: 10.1152/jn.00542.2014. Epub 2014 Oct 15. J Neurophysiol. 2015. PMID: 25343784 Free PMC article.
-
Role of the dorsal medulla in the neurogenesis of airway protection.Pulm Pharmacol Ther. 2015 Dec;35:105-10. doi: 10.1016/j.pupt.2015.10.012. Epub 2015 Nov 5. Pulm Pharmacol Ther. 2015. PMID: 26549786 Free PMC article. Review.
References
-
- Baekey D. M., Morris K. F., Nuding S. C., Segers L. S., Lindsey B. G., Shannon R. (2003). Medullary raphé neuron activity is altered during fictive cough in the decerebrate cat. J. Appl. Physiol. 94, 93–100 - PubMed
-
- Benjamini Y., Hochberg Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Series B Stat. Methodol. 57, 289–300
-
- Bolser D. C. (1991). Fictive cough in the cat. J. Appl. Physiol. 71, 2325–2331 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

