Poxvirus cell entry: how many proteins does it take?
- PMID: 22754644
- PMCID: PMC3386626
- DOI: 10.3390/v4050688
Poxvirus cell entry: how many proteins does it take?
Abstract
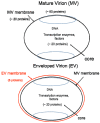
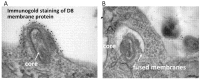
For many viruses, one or two proteins enable cell binding, membrane fusion and entry. The large number of proteins employed by poxviruses is unprecedented and may be related to their ability to infect a wide range of cells. There are two main infectious forms of vaccinia virus, the prototype poxvirus: the mature virion (MV), which has a single membrane, and the extracellular enveloped virion (EV), which has an additional outer membrane that is disrupted prior to fusion. Four viral proteins associated with the MV membrane facilitate attachment by binding to glycosaminoglycans or laminin on the cell surface, whereas EV attachment proteins have not yet been identified. Entry can occur at the plasma membrane or in acidified endosomes following macropinocytosis and involves actin dynamics and cell signaling. Regardless of the pathway or whether the MV or EV mediates infection, fusion is dependent on 11 to 12 non-glycosylated, transmembrane proteins ranging in size from 4- to 43-kDa that are associated in a complex. These proteins are conserved in poxviruses making it likely that a common entry mechanism exists. Biochemical studies support a two-step process in which lipid mixing of viral and cellular membranes is followed by pore expansion and core penetration.
Keywords: endocytosis; macropinocytosis; transmembrane proteins; vaccinia virus entry; viral membrane fusion.
Figures


References
-
- Moss B. Poxviridae: The Viruses and Their Replication. In: Knipe D.M., Howley P.M., editors. Fields Virology. Vol. 2. Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2007. pp. 2905–2946.
-
- Moss B. Poxvirus entry and membrane fusion. Virology. 2006;344:48–54. - PubMed
-
- Moss B., de Silva F. Poxvirus DNA Replication and Human Disease. In: DePamphilis M.L., editor. DNA Replication & Human Disease. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY, USA: 2006. pp. 707–727.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

