Initiating head development in mouse embryos: integrating signalling and transcriptional activity
- PMID: 22754658
- PMCID: PMC3382960
- DOI: 10.1098/rsob.120030
Initiating head development in mouse embryos: integrating signalling and transcriptional activity
Abstract
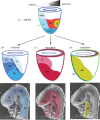
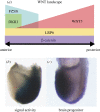
The generation of an embryonic body plan is the outcome of inductive interactions between the progenitor tissues that underpin their specification, regionalization and morphogenesis. The intercellular signalling activity driving these processes is deployed in a time- and site-specific manner, and the signal strength must be precisely controlled. Receptor and ligand functions are modulated by secreted antagonists to impose a dynamic pattern of globally controlled and locally graded signals onto the tissues of early post-implantation mouse embryo. In response to the WNT, Nodal and Bone Morphogenetic Protein (BMP) signalling cascades, the embryo acquires its body plan, which manifests as differences in the developmental fate of cells located at different positions in the anterior-posterior body axis. The initial formation of the anterior (head) structures in the mouse embryo is critically dependent on the morphogenetic activity emanating from two signalling centres that are juxtaposed with the progenitor tissues of the head. A common property of these centres is that they are the source of antagonistic factors and the hub of transcriptional activities that negatively modulate the function of WNT, Nodal and BMP signalling cascades. These events generate the scaffold of the embryonic head by the early-somite stage of development. Beyond this, additional tissue interactions continue to support the growth, regionalization, differentiation and morphogenesis required for the elaboration of the structure recognizable as the embryonic head.
Keywords: gene transcription; head formation; morphogenesis; mouse embryo; signalling.
Figures
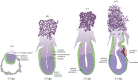


References
-
- Rossant J, Tam PP. 2004. Emerging asymmetry and embryonic patterning in early mouse development. Dev. Cell 7, 155–164 10.1016/j.devcel.2004.07.012 (doi:10.1016/j.devcel.2004.07.012) - DOI - PubMed
-
- Tam PP, Gad JM. 2004. Gastrulation of the mouse embryo. In Gastrulation (ed. Stern C. D.), pp. 223–262 Cold Spring Harbor, NY: Cold Spring Harbour Laboratory Press
-
- Tam PP, Loebel DA. 2007. Gene function in mouse embryogenesis: get set for gastrulation. Nat. Rev. Genet. 8, 368–381 10.1038/nrg2084 (doi:10.1038/nrg2084) - DOI - PubMed
-
- Tam PP. 1989. Regionalisation of the mouse embryonic ectoderm: allocation of prospective ectodermal tissues during gastrulation. Development 107, 55–67 - PubMed
-
- Cajal M, Lawson KA, Hill B, Moreau A, Rao J, Ross A, Collignon J, Camus A. 2012. Clonal and molecular analysis of the prospective anterior neural boundary in the mouse embryo. Development 139, 423–436 10.1242/dev.075499 (doi:10.1242/dev.075499) - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
