Automated measurement of uptake in cerebellum, liver, and aortic arch in full-body FDG PET/CT scans
- PMID: 22755696
- PMCID: PMC3365916
- DOI: 10.1118/1.4711815
Automated measurement of uptake in cerebellum, liver, and aortic arch in full-body FDG PET/CT scans
Abstract
Purpose: The purpose of this work was to develop and validate fully automated methods for uptake measurement of cerebellum, liver, and aortic arch in full-body PET/CT scans. Such measurements are of interest in the context of uptake normalization for quantitative assessment of metabolic activity and/or automated image quality control.
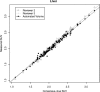
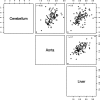
Methods: Cerebellum, liver, and aortic arch regions were segmented with different automated approaches. Cerebella were segmented in PET volumes by means of a robust active shape model (ASM) based method. For liver segmentation, a largest possible hyperellipsoid was fitted to the liver in PET scans. The aortic arch was first segmented in CT images of a PET/CT scan by a tubular structure analysis approach, and the segmented result was then mapped to the corresponding PET scan. For each of the segmented structures, the average standardized uptake value (SUV) was calculated. To generate an independent reference standard for method validation, expert image analysts were asked to segment several cross sections of each of the three structures in 134 F-18 fluorodeoxyglucose (FDG) PET/CT scans. For each case, the true average SUV was estimated by utilizing statistical models and served as the independent reference standard.
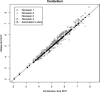
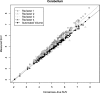
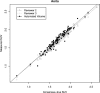
Results: For automated aorta and liver SUV measurements, no statistically significant scale or shift differences were observed between automated results and the independent standard. In the case of the cerebellum, the scale and shift were not significantly different, if measured in the same cross sections that were utilized for generating the reference. In contrast, automated results were scaled 5% lower on average although not shifted, if FDG uptake was calculated from the whole segmented cerebellum volume. The estimated reduction in total SUV measurement error ranged between 54.7% and 99.2%, and the reduction was found to be statistically significant for cerebellum and aortic arch.
Conclusions: With the proposed methods, the authors have demonstrated that automated SUV uptake measurements in cerebellum, liver, and aortic arch agree with expert-defined independent standards. The proposed methods were found to be accurate and showed less intra- and interobserver variability, compared to manual analysis. The approach provides an alternative to manual uptake quantification, which is time-consuming. Such an approach will be important for application of quantitative PET imaging to large scale clinical trials.
© 2012 American Association of Physicists in Medicine.
Figures



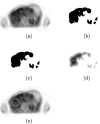





References
-
- Li C., Wang X., Xia Y., Eberl S., Yin Y., and Feng D. D., “Automated PET-guided liver segmentation from low-contrast CT volumes using probabilistic atlas,” Comput. Methods Programs Biomed. 3565–3568 (2011). - PubMed
-
- Heimann T., van Ginneken B., Styner M., Arzhaeva Y., Aurich V., Bauer C., Beck A., Becker C., Beichel R., Bekes G., Bello F., Binnig G., Bischof H., Bornik A., Cashman P., Chi Y., Cordova A., Dawant B., Fidrich M., Furst J., Furukawa D., Grenacher L., Hornegger J., Kainmuller D., Kitney R., Kobatake H., Lamecker H., Lange T., Lee J., Lennon B., Li R., Li S., Meinzer H. P., Nemeth G., Raicu D., Rau A. M., van Rikxoort E., Rousson M., Rusko L., Saddi K., Schmidt G., Seghers D., Shimizu A., Slagmolen P., Sorantin E., Soza G., Susomboon R., Waite J., Wimmer A., and Wolf I., “Comparison and evaluation of methods for liver segmentation from CT datasets,” IEEE Trans. Med. Imaging 28(8), 1251–1265 (2009).10.1109/TMI.2009.2013851 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

