O-GlcNAcylation and oxidation of proteins: is signalling in the cardiovascular system becoming sweeter?
- PMID: 22757958
- PMCID: PMC3389386
- DOI: 10.1042/CS20110638
O-GlcNAcylation and oxidation of proteins: is signalling in the cardiovascular system becoming sweeter?
Abstract
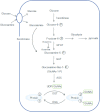
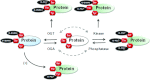
O-GlcNAcylation is an unusual form of protein glycosylation, where a single-sugar [GlcNAc (N-acetylglucosamine)] is added (via β-attachment) to the hydroxyl moiety of serine and threonine residues of nuclear and cytoplasmic proteins. A complex and extensive interplay exists between O-GlcNAcylation and phosphorylation. Many phosphorylation sites are also known glycosylation sites, and this reciprocal occupancy may produce different activities or alter the stability in a target protein. The interplay between these two post-translational modifications is not always reciprocal, as some proteins can be concomitantly phosphorylated and O-GlcNAcylated, and the adjacent phosphorylation or O-GlcNAcylation can regulate the addition of either moiety. Increased cardiovascular production of ROS (reactive oxygen species), termed oxidative stress, has been consistently reported in various chronic diseases and in conditions where O-GlcNAcylation has been implicated as a contributing mechanism for the associated organ injury/protection (for example, diabetes, Alzheimer's disease, arterial hypertension, aging and ischaemia). In the present review, we will briefly comment on general aspects of O-GlcNAcylation and provide an overview of what has been reported for this post-translational modification in the cardiovascular system. We will then specifically address whether signalling molecules involved in redox signalling can be modified by O-GlcNAc (O-linked GlcNAc) and will discuss the critical interplay between O-GlcNAcylation and ROS generation. Experimental evidence indicates that the interactions between O-GlcNAcylation and oxidation of proteins are important not only for cell regulation in physiological conditions, but also under pathological states where the interplay may become dysfunctional and thereby exacerbate cellular injury.
Figures

Similar articles
-
O-linked beta-N-acetylglucosamine (O-GlcNAc): Extensive crosstalk with phosphorylation to regulate signaling and transcription in response to nutrients and stress.Biochim Biophys Acta. 2010 Feb;1800(2):96-106. doi: 10.1016/j.bbagen.2009.07.018. Epub 2009 Aug 6. Biochim Biophys Acta. 2010. PMID: 19647786 Free PMC article. Review.
-
Modification of histones by sugar β-N-acetylglucosamine (GlcNAc) occurs on multiple residues, including histone H3 serine 10, and is cell cycle-regulated.J Biol Chem. 2011 Oct 28;286(43):37483-95. doi: 10.1074/jbc.M111.284885. Epub 2011 Sep 6. J Biol Chem. 2011. PMID: 21896475 Free PMC article.
-
O-GlcNAc signaling in the cardiovascular system.Circ Res. 2010 Jul 23;107(2):171-85. doi: 10.1161/CIRCRESAHA.110.224675. Circ Res. 2010. PMID: 20651294 Free PMC article. Review.
-
O-GlcNAcylation: a novel pathway contributing to the effects of endothelin in the vasculature.Am J Physiol Regul Integr Comp Physiol. 2011 Feb;300(2):R236-50. doi: 10.1152/ajpregu.00230.2010. Epub 2010 Nov 10. Am J Physiol Regul Integr Comp Physiol. 2011. PMID: 21068200 Free PMC article. Review.
-
[Protein O-GlcNAcylation and regulation of cell signalling: involvement in pathophysiology].Biol Aujourdhui. 2014;208(2):109-17. doi: 10.1051/jbio/2014015. Epub 2014 Sep 8. Biol Aujourdhui. 2014. PMID: 25190571 Review. French.
Cited by
-
Protein O-GlcNAcylation in Metabolic Modulation of Skeletal Muscle: A Bright but Long Way to Go.Metabolites. 2022 Sep 22;12(10):888. doi: 10.3390/metabo12100888. Metabolites. 2022. PMID: 36295790 Free PMC article. Review.
-
Pathogenesis of chronic hyperglycemia: from reductive stress to oxidative stress.J Diabetes Res. 2014;2014:137919. doi: 10.1155/2014/137919. Epub 2014 Jun 16. J Diabetes Res. 2014. PMID: 25019091 Free PMC article. Review.
-
Non-canonical Interaction Between O-Linked N-Acetylglucosamine Transferase and miR-146a-5p Aggravates High Glucose-Induced Endothelial Inflammation.Front Physiol. 2020 Oct 30;11:1091. doi: 10.3389/fphys.2020.01091. eCollection 2020. Front Physiol. 2020. PMID: 33192537 Free PMC article.
-
O-GlcNAcylation: the "stress and nutrition receptor" in cell stress response.Cell Stress Chaperones. 2021 Mar;26(2):297-309. doi: 10.1007/s12192-020-01177-y. Epub 2020 Nov 7. Cell Stress Chaperones. 2021. PMID: 33159661 Free PMC article. Review.
-
O-GlcNAc Modification During Pregnancy: Focus on Placental Environment.Front Physiol. 2018 Sep 12;9:1263. doi: 10.3389/fphys.2018.01263. eCollection 2018. Front Physiol. 2018. PMID: 30298013 Free PMC article.
References
-
- Varki A., Cummings R. D., Esko J. D., Freeze H. H., Stanley P., Bertozzi C. R., Hart G. W., Etzler M. E. Essentials of Glycobiology. 2nd edition. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2009. - PubMed
-
- Taylor M. E., Drickamer K. Introduction to Glycobiology. 2nd edition. Oxford: Oxford University Press; 2006.
-
- Spiro R. G. Protein glycosylation: nature, distribution, enzymatic formation, and disease implications of glycopeptide bonds. Glycobiology. 2002;12:43R–56R. - PubMed
-
- Torres C. R., Hart G. W. Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J. Biol. Chem. 1984;259:3308–3317. - PubMed
-
- Kelly W. G., Hart G. W. Glycosylation of chromosomal proteins: localization of O-linked N-acetylglucosamine in Drosophila chromatin. Cell. 1989;57:243–251. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

