Iron-catalyzed alkylations of aryl sulfamates and carbamates
- PMID: 22758657
- PMCID: PMC3479317
- DOI: 10.1021/ol301681z
Iron-catalyzed alkylations of aryl sulfamates and carbamates
Abstract
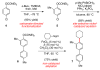
The alkylation of aryl sulfamates and carbamates using iron catalysis is reported. The method constructs sp(2)-sp(3) carbon-carbon bonds and provides synthetically useful yields across a range of substrates (>35 examples). The directing group ability of sulfamates and carbamates, accompanied by their low reactivity toward conventional cross-couplings, renders these substrates useful for the synthesis of polyfunctionalized arenes.
Figures
References
-
- Kharasch MS, Fields EK. J. Am. Chem. Soc. 1941;63:2316.
-
- Tamura M, Kochi JK. J. Am. Chem. Soc. 1971;93:1487.
- Smith RS, Kochi JK. J. Org. Chem. 1976;41:502.
-
-
For the iron-catalyzed alkylation of aryl halides, tosylates, and triflates, see: Fürstner A, Leitner A. Angew. Chem. Int. Ed. 2002;41:609. Fürstner A, Leitner A, Méndez M, Krause H. J. Am. Chem. Soc. 2002;124:13856. Fürstner A, Martin R, Krause H, Günter S, Goddard R, Lehmann CW. J. Am. Chem. Soc. 2008;130:8773. For the iron-catalyzed alkylation of heteroaromatic sulfonates and phosphates, see: Gøgsig TM, Lindhardt AT, Skrydstrup T. Org. Lett. 2009;11:4886. For a relevant mechanistic study, see: Kleimark J, Larsson P-F, Emamy P, Hedström A, Norrby P-O. Adv. Synth. Catal. 2012;354:448.
-
-
-
For the related iron-catalyzed alkylation of styrenyl or electrondeficient vinyl pivalates, see: Li B-J, Xu L, Wu Z-H, Guan B-T, Sun C-L, Wang B-Q, Shi Z-J. J. Am. Chem. Soc. 2009;131:14656.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical