Transmembrane domains are critical to the interaction between platelet glycoprotein V and glycoprotein Ib-IX complex
- PMID: 22759073
- PMCID: PMC3499136
- DOI: 10.1111/j.1538-7836.2012.04841.x
Transmembrane domains are critical to the interaction between platelet glycoprotein V and glycoprotein Ib-IX complex
Abstract
Background: The glycoprotein (GP) Ib-IX-V complex, the von Willebrand factor receptor on the platelet surface, is critically involved in hemostasis and thrombosis. The GPV subunit interacts with GPIb-IX to form the GPIb-IX-V complex, but the underlying molecular basis remains unclear. It was observed earlier that efficient expression of GPV in the plasma membrane requires co-expression of GPIb-IX.
Objectives and methods: Hypothesizing that GPIb-IX stabilizes GPV through direct interaction and consequently enhances GPV surface expression, we aim in this study to identify structural elements in the complex that mediate the interaction between GPV and GPIb-IX by analyzing mutational effects on GPV surface expression in transfected Chinese hamster ovary cells.
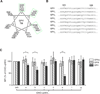
Results: Enhancement of GPV surface expression by GPIb-IX requires transmembrane domains of both GPV and GPIbα, as replacing the GPV transmembrane domain with an unrelated poly-leucine-alanine sequence abolished the enhancing effect of GPIb-IX. Additional mutagenesis analysis of the GPV transmembrane helix identified three helical sides containing conserved polar residues as critical to efficient GPV surface expression. Similarly, replacing residues in three sides (Gly495/Ala502/Leu509, Phe491/Trp498/Val505, and Y492/L499/L506) of the GPIbα transmembrane domain with leucines preserved the surface expression level of GPIb-IX but significantly altered that of GPV.
Conclusions: Our results demonstrate for the first time the importance of transmembrane domains for efficient surface expression of GPV and suggest that GPV and GPIbα transmembrane domains interact with each other, contributing to assembly of the GPIb-IX-V complex.
© 2012 International Society on Thrombosis and Haemostasis.
Conflict of interest statement
The authors state that they have no conflict of interest.
Figures





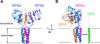
References
-
- Lopez JA. The platelet glycoprotein Ib-IX complex. Blood Coagul. Fibrinolysis. 1994;5:97–119. - PubMed
-
- Berndt MC, Shen Y, Dopheide SM, Gardiner EE, Andrews RK. The vascular biology of the glycoprotein Ib-IX-V complex. Thromb. Haemost. 2001;86:178–188. - PubMed
-
- Andrews RK, Shen Y, Gardiner EE, Dong JF, Lopez JA, Berndt MC. The glycoprotein Ib-IX-V complex in platelet adhesion and signaling. Thromb. Haemost. 1999;82:357–364. - PubMed
-
- Andrews RK, Gardiner EE, Shen Y, Whisstock JC, Berndt MC. Glycoprotein Ib-IX-V. Int. J. Biochem. Cell Biol. 2003;35:1170–1174. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

