Recombinant production of self-assembling β-structured peptides using SUMO as a fusion partner
- PMID: 22759375
- PMCID: PMC3512519
- DOI: 10.1186/1475-2859-11-92
Recombinant production of self-assembling β-structured peptides using SUMO as a fusion partner
Abstract
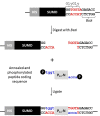
Background: Self-assembling peptides that form nanostructured hydrogels are important biomaterials for tissue engineering scaffolds. The P₁₁-family of peptides includes, P₁₁-4 (QQRFEWEFEQQ) and the complementary peptides P₁₁-13 (EQEFEWEFEQE) and P₁₁-14 (QQOrnFOrnWOrnFOrnQQ). These form self-supporting hydrogels under physiological conditions (pH 7.4, 140 mM NaCl) either alone (P₁₁-4) or when mixed (P₁₁-13 and P₁₁-14). We report a SUMO-peptide expression strategy suitable for allowing release of native sequence peptide by SUMO protease cleavage.
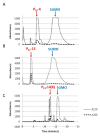
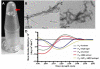
Results: We have expressed SUMO-peptide fusion proteins from pET vectors by using autoinduction methods. Immobilised metal affinity chromatography was used to purify the fusion protein, followed by SUMO protease cleavage in water to release the peptides, which were recovered by reverse phase HPLC. The peptide samples were analysed by electrospray mass spectrometry and self-assembly was followed by circular dichroism and transmission electron microscopy.
Conclusions: The fusion proteins were produced in high yields and the β-structured peptides were efficiently released by SUMO protease resulting in peptides with no additional amino acid residues and with recoveries of 46% to 99%. The peptides behaved essentially the same as chemically synthesised and previously characterised recombinant peptides in self-assembly and biophysical assays.
Figures

References
-
- Bell CJ, Carrick LM, Katta J, Jin Z, Ingham E, Aggeli A, Boden N, Waigh TA, Fisher J. Self-assembling peptides as injectable lubricants for osteoarthritis. J Biomed Mater Res A. 2006;78:236–246. - PubMed
-
- Firth A, Aggeli A, Burke JL, Yang XB, Kirkham J. Biomimetic self-assembling peptides as injectable scaffolds for hard tissue engineering. Nanomedicine. 2006;1:189–199. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

