Exposure to benzo[a]pyrene of Hepatic Cytochrome P450 Reductase Null (HRN) and P450 Reductase Conditional Null (RCN) mice: Detection of benzo[a]pyrene diol epoxide-DNA adducts by immunohistochemistry and 32P-postlabelling
- PMID: 22759596
- PMCID: PMC7477777
- DOI: 10.1016/j.toxlet.2012.06.016
Exposure to benzo[a]pyrene of Hepatic Cytochrome P450 Reductase Null (HRN) and P450 Reductase Conditional Null (RCN) mice: Detection of benzo[a]pyrene diol epoxide-DNA adducts by immunohistochemistry and 32P-postlabelling
Abstract
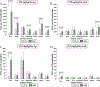
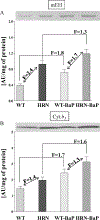
Benzo[a]pyrene (BaP) is a widespread environmental carcinogen activated by cytochrome P450 (P450) enzymes. In Hepatic P450 Reductase Null (HRN) and Reductase Conditional Null (RCN) mice, P450 oxidoreductase (Por) is deleted specifically in hepatocytes, resulting in the loss of essentially all hepatic P450 function. Treatment of HRN mice with a single i.p. or oral dose of BaP (12.5 or 125mg/kg body weight) resulted in higher DNA adduct levels in liver (up to 10-fold) than in wild-type (WT) mice, indicating that hepatic P450s appear to be more important for BaP detoxification in vivo. Similar results were obtained in RCN mice. We tested whether differences between hepatocytes and non-hepatocytes in P450 activity may underlie the increased liver BaP-DNA binding in HRN mice. Cellular localisation by immunohistochemistry of BaP-DNA adducts showed that HRN mice have ample capacity for formation of BaP-DNA adducts in liver, indicating that the metabolic process does not result in the generation of a reactive species different from that formed in WT mice. However, increased protein expression of cytochrome b(5) in hepatic microsomes of HRN relative to WT mice suggests that cytochrome b(5) may modulate the P450-mediated bioactivation of BaP in HRN mice, partially substituting the function of Por.
Copyright © 2012 Elsevier Ireland Ltd. All rights reserved.
Conflict of interest statement
Conflict of interest
None declared.
Figures


References
-
- Arlt VM, Henderson CJ, Wolf CR, Schmeiser HH, Phillips DH, Stiborova M, 2006. Bioactivation of 3-aminobenzanthrone, a human metabolite of the environmental pollutant 3-nitrobenzanthrone: evidence for DNA adduct formation mediated by cytochrome P450 enzymes and peroxidases. Cancer Letters 234, 220–231. - PubMed
-
- Arlt VM, Singh R, Stiborova M, Gamboa da Costa G, Frei E, Evans JD, Farmer PB, Wolf CR, Henderson CJ, Phillips DH, 2011. Effect of hepatic cytochrome P450 (P450) oxidoreductase deficiency on 2-amino-1-methyl6-phenylimidazo[4,5-b]pyridine-DNA adduct formation in P450 reductase conditional null mice. Drug Metabolism and Disposition 39, 2169–2173. - PubMed
-
- Arlt VM, Stiborova M, Henderson CJ, Osborne MR, Bieler CA, Frei E, Martinek V, Sopko B, Wolf CR, Schmeiser HH, Phillips DH, 2005. Environmental pollutant and potent mutagen 3-nitrobenzanthrone forms DNA adducts after reduction by NAD(P)H:quinone oxidoreductase and conjugation by acetyltransferases and sulfotransferases in human hepatic cytosols. Cancer Research 65, 2644–2652. - PubMed
-
- Arlt VM, Stiborova M, Henderson CJ, Thiemann M, Frei E, Aimova D, Singh R, Gamboa da Costa G, Schmitz OJ, Farmer PB, Wolf CR, Phillips DH, 2008. Metabolic activation of benzo[a]pyrene invitro by hepatic cytochrome P450 contrasts with detoxification in vivo: experiments with hepatic cytochrome P450 reductase null mice. Carcinogenesis 29, 656–665. - PubMed
-
- Baird WM, Hooven LA, Mahadevan B, 2005. Carcinogenic polycyclic aromatic hydrocarbon-DNA adducts and mechanism of action.Environmental and Molecular Mutagenesis 45, 106–114. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

