Inhibition of Na(+)-K(+)-2Cl(-) cotransporter isoform 1 accelerates temozolomide-mediated apoptosis in glioblastoma cancer cells
- PMID: 22759954
- PMCID: PMC3603147
- DOI: 10.1159/000339047
Inhibition of Na(+)-K(+)-2Cl(-) cotransporter isoform 1 accelerates temozolomide-mediated apoptosis in glioblastoma cancer cells
Abstract
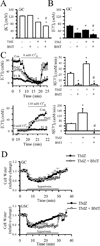
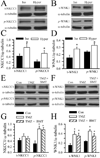
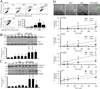
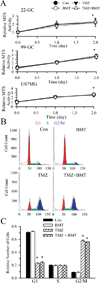
The hallmark of apoptosis is a significant reduction in cell volume (AVD) resulting from loss of K(+)(i) and Cl(-)(i). Loss of cell volume and lowering of ionic strength of intracellular K(+) and Cl(-) occur before any other detectable characteristics of apoptosis. In the present study, temozolomide (TMZ) triggered loss of K(+)(i) and Cl(-)(i) and AVD in primary glioblastoma multiforme (GBM) cancer cells (GC) and GC cancer stem cells (GSC). We hypothesize that Na(+)-K(+)-2Cl(-) cotransporter isoform 1 (NKCC1) counteracts AVD during apoptosis in GBM cancer cells by regulating cell volume and Cl(-) homeostasis. NKCC1 protein was expressed in both GC and GSC and played an essential role in regulatory volume increase (RVI) in response to hypertonic cell shrinkage and isotonic cell shrinkage. Blocking NKCC1 activity with its potent inhibitor bumetanide abolished RVI. These cells maintained a basal [Cl(-)](i) (~ 68 mM) above the electrochemical equilibrium for Cl(-)(i). NKCC1 also functioned to replenish Cl(-)(i) levels following the loss of Cl(-)(i). TMZ-treated cells exhibited increased phosphorylation of NKCC1 and its up-stream novel Cl(-)/volume-sensitive regulatory kinase WNK1. Inhibition of NKCC1 activity with bumetanide accelerated AVD, early apoptosis, as well as activation of caspase-3 and caspase-8. Taken together, this study strongly suggests that NKCC1 is an essential mechanism in GBM cells to maintain K(+), Cl(-), and volume homeostasis to counteract TMZ-induced loss of K(+), Cl(-) and AVD. Therefore, blocking NKCC1 function augments TMZ-induced apoptosis in glioma cells.
Copyright © 2012 S. Karger AG, Basel.
Figures


References
-
- Louis DN. Molecular pathology of malignant gliomas. Annu Rev Pathol. 2006;1:97–117. - PubMed
-
- Denysenko T, Gennero L, Roos MA, Melcarne A, Juenemann C, Faccani G, Morra I, Cavallo G, Reguzzi S, Pescarmona G, Ponzetto A. Glioblastoma cancer stem cells: heterogeneity, microenvironment and related therapeutic strategies. Cell Biochem Funct. 2010;28:343–351. - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
