Voltage-sensing phosphatase reveals temporal regulation of TRPC3/C6/C7 channels by membrane phosphoinositides
- PMID: 22760061
- PMCID: PMC3431592
- DOI: 10.4161/chan.20883
Voltage-sensing phosphatase reveals temporal regulation of TRPC3/C6/C7 channels by membrane phosphoinositides
Abstract
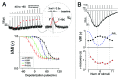
TRPC3/C6/C7 channels, a subgroup of classical/canonical TRP channels, are activated by diacylglycerol produced via activation of phospholipase C (PLC)-coupled receptors. Recognition of the physiological importance of these channels has been steadily growing, but the mechanism by which they are regulated remains largely unknown. We recently used a membrane-resident danio rerio voltage-sensing phosphatase (DrVSP) to study TRPC3/C6/C7 regulation and found that the channel activity was controlled by PtdIns(4,5)P(2)-DAG signaling in a self-limiting manner (Imai Y et al., the Journal of Physiology, 2012). In this addendum, we present the advantages of using DrVSP as a molecular tool to study PtdIns(4,5)P(2) regulation. DrVSP should be readily applicable for studying phosphoinositide metabolism-linked channel regulation as well as lipid dynamics. Furthermore, in comparison to other modes of self-limiting ion channel regulation, the regulation of TRPC3/C6/C7 channels seems highly susceptible to activation signal strength, which could potentially affect both open duration and the time to peak activation and inactivation. Dysfunction of such self-limiting regulation may contribute to the pathology of the cardiovascular system, gastrointestinal tract and brain, as these channels are broadly distributed and affected by numerous neurohormonal agonists.
Figures

Comment on
- Imai Y, Itsuki K, Okamura Y, Inoue R, Mori MX. A self-limiting regulation of vasoconstrictor-activated TRPC3/C6/C7 channels coupled to PI(4,5)P₂-diacylglycerol signalling. J Physiol. 2012;590:1101–19. doi: 10.1113/jphysiol.2011.221358. doi: 10.1113/jphysiol.2011.221358
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
