CD24 controls Src/STAT3 activity in human tumors
- PMID: 22760497
- PMCID: PMC11114558
- DOI: 10.1007/s00018-012-1055-9
CD24 controls Src/STAT3 activity in human tumors
Abstract
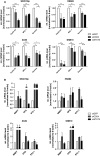
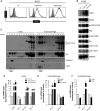
CD24 is a glycosyl-phosphatidylinositol-anchored membrane protein that is frequently over-expressed in a variety of human carcinomas and is correlated with poor prognosis. In cancer cell lines, changes of CD24 expression can alter several cellular properties in vitro and tumor growth in vivo. However, little is known about how CD24 mediates these effects. Here we have analyzed the functional consequences of CD24 knock-down or over-expression in human cancer cell lines. Depletion of CD24 reduced cell proliferation and adhesion, enhanced apoptosis, and regulated the expression of various genes some of which were identified as STAT3 target genes. Loss of CD24 reduced STAT3 and FAK phosphorylation. Diminished STAT3 activity was confirmed by specific reporter assays. We found that reduced STAT3 activity after CD24 knock-down was accompanied by altered Src phosphorylation. Silencing of Src, similar to CD24, targeted the expression of prototype STAT3-regulated genes. Likewise, the over-expression of CD24 augmented Src-Y416 phosphorylation, the recruitment of Src into lipid rafts and the expression of STAT3-dependent target genes. An antibody to CD24 was effective in reducing tumor growth of A549 lung cancer and BxPC3 pancreatic cancer xenografts in mice. Antibody treatment affected the level of Src-phosphorylation in the tumor and altered the expression of STAT3 target genes. Our results provide evidence that CD24 regulates STAT3 and FAK activity and suggest an important role of Src in this process. Finally, the targeting of CD24 by antibodies could represent a novel route for tumor therapy.
Figures






Similar articles
-
Antibody-based targeting of CD24 enhances antitumor effect of cetuximab via attenuating phosphorylation of Src/STAT3.Biomed Pharmacother. 2017 Jun;90:427-436. doi: 10.1016/j.biopha.2017.03.094. Epub 2017 Apr 6. Biomed Pharmacother. 2017. PMID: 28391164
-
CD24 interacts with and promotes the activity of c-src within lipid rafts in breast cancer cells, thereby increasing integrin-dependent adhesion.Cell Mol Life Sci. 2012 Feb;69(3):435-48. doi: 10.1007/s00018-011-0756-9. Epub 2011 Jun 28. Cell Mol Life Sci. 2012. PMID: 21710320 Free PMC article.
-
Evaluation of STAT3 signaling in ALDH+ and ALDH+/CD44+/CD24- subpopulations of breast cancer cells.PLoS One. 2013 Dec 23;8(12):e82821. doi: 10.1371/journal.pone.0082821. eCollection 2013. PLoS One. 2013. PMID: 24376586 Free PMC article.
-
From mechanism to therapy: the journey of CD24 in cancer.Front Immunol. 2024 May 31;15:1401528. doi: 10.3389/fimmu.2024.1401528. eCollection 2024. Front Immunol. 2024. PMID: 38881902 Free PMC article. Review.
-
Decoding CD24: Roles of chemoradiotherapy resistance and potential as therapeutic targets.Oncol Res. 2025 May 29;33(6):1347-1361. doi: 10.32604/or.2025.059327. eCollection 2025. Oncol Res. 2025. PMID: 40486886 Free PMC article. Review.
Cited by
-
Emerging role of cancer stem cells in the biology and treatment of ovarian cancer: basic knowledge and therapeutic possibilities for an innovative approach.J Exp Clin Cancer Res. 2013 Aug 1;32(1):48. doi: 10.1186/1756-9966-32-48. J Exp Clin Cancer Res. 2013. PMID: 23902592 Free PMC article. Review.
-
Checkpoint CD24 function on tumor and immunotherapy.Front Immunol. 2024 Feb 29;15:1367959. doi: 10.3389/fimmu.2024.1367959. eCollection 2024. Front Immunol. 2024. PMID: 38487533 Free PMC article. Review.
-
Emerging Immune Checkpoint Molecules on Cancer Cells: CD24 and CD200.Int J Mol Sci. 2023 Oct 11;24(20):15072. doi: 10.3390/ijms242015072. Int J Mol Sci. 2023. PMID: 37894750 Free PMC article. Review.
-
CD24 associates with EGFR and supports EGF/EGFR signaling via RhoA in gastric cancer cells.J Transl Med. 2016 Feb 1;14:32. doi: 10.1186/s12967-016-0787-y. J Transl Med. 2016. PMID: 26830684 Free PMC article.
-
CD24 flags anastasis in melanoma cells.Apoptosis. 2025 Feb;30(1-2):1-15. doi: 10.1007/s10495-024-01990-1. Epub 2024 Aug 13. Apoptosis. 2025. PMID: 39136818 Free PMC article.
References
-
- Kay R, Rosten PM, Humphries RK. CD24, a signal transducer modulating B cell activation responses, is a very short peptide with a glycosyl phosphatidylinositol membrane anchor. J Immunol. 1991;147(4):1412–1416. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

