A randomized clinical trial of trans-dermal nicotine replacement in pregnant African-American smokers
- PMID: 22761006
- PMCID: PMC3679235
- DOI: 10.1007/s10995-012-1069-9
A randomized clinical trial of trans-dermal nicotine replacement in pregnant African-American smokers
Abstract
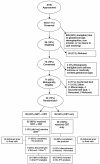
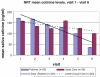
We compared acceptability, adherence and efficacy of trans-dermal nicotine patches and cognitive behavioral therapy (Group 1) to cognitive behavioral therapy alone (Group 2) in minority pregnant smokers. This is a randomized controlled trial. 52 women were recruited during pregnancy with a mean gestational age 18.5 ± 5.0 weeks and followed through delivery. Randomization was by site and initial cotinine levels. Interventionists and interviewers were blinded to group assignment. Two different nicotine replacement therapy dosing regiments were administered according to the baseline salivary cotinine level. A process evaluation model summarized patient adherence. The main outcome measure was self-report of cessation since last visit, confirmed by exhaled carbon monoxide. Analyses of categorical and continuous measures were conducted as well as linear trend tests of salivary cotinine levels. Women lost to follow-up were considered treatment failures. Participants were on average 27.5 ± 5.4 years old, 81 % were single, 69 % unemployed and 96 % were Medicaid eligible. A process evaluation indicated patients in both groups were adherent to scheduled program procedures through Visit 4, but not for Visits 5 and 6. Confirmed quit rates were: at visit 3, 23 (Group 1) and 0 % (Group 2) (p = 0.02); at visits 4 and 5, no difference; at visit 6, 19 (Group 1) and 0 % (Group 2) (p = 0.05). Group 1 delivered infants with a mean gestational age of 39.4 weeks versus 38.4 weeks in Group 2 (p = 0.02). 73 % (52/71) of the eligible smokers agreed to participate and 65 % (17/26) of Group 1 completed the protocol (i.e. attended 6 visits). A comparison of Group 1 and 2 quit rates confirmed a non-significant difference.
Trial registration: ClinicalTrials.gov NCT00341432.
Figures
References
-
- Simpson WJ. A preliminary report on cigarette smoking and the incidence of prematurity. American Journal of Obstetrics and Gynecology. 1957;73:807–815. - PubMed
-
- Mohsin M, Jalaludin B. Influence of previous pregnancy outcomes and continued smoking on subsequent pregnancy outcomes: an exploratory study in Australia. BJOG: An International Journal of Obstetrics & Gynaecology. 2008;115:1428–1435. - PubMed
-
- U.S. Department of Health and Human Services . How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and health Promotion, Office on Smoking and Health; Atlanta, GA: 2010. Retrieved from http://www.surgeongeneral.gov/library/tobaccosmoke/report/full_report.pdf.
-
- National Center for Health Statistics . Health, United States, 2009: With Special Feature on Medical Technology. Hyattsville, MD: 2010. Retreived from http://www.cdc.gov/nchs/data/hus/hus09.pdf. - PubMed
-
- Fiore MC, Bailey WC, Cohen SJ, Dorfman SF, Goldstein MG, Gritz ER. Treating tobacco use and dependence: Clinical practice guideline. US-DHHS, Public Health Service; Rockville, MD: 2000.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

