The Tellurium compound, AS101, increases SIRT1 level and activity and prevents type 2 diabetes
- PMID: 22761194
- PMCID: PMC3409680
- DOI: 10.18632/aging.100468
The Tellurium compound, AS101, increases SIRT1 level and activity and prevents type 2 diabetes
Abstract
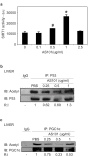
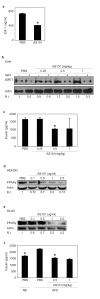
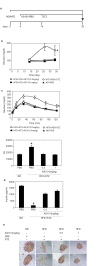
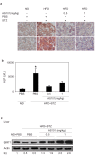
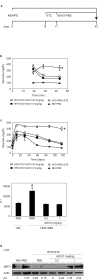
The histone deacetylase, SIRT1, plays a major role in glucose regulation and lipid metabolism. Ammonium Trichloro (dioxoethylene-o,o') Tellurate, AS101, is a potent in vitro and in vivo immunomodulator, with several potential therapeutic applications. AS101 administration resulted in upregulation of SIRT1 protein expression and activity. These effects were associated with decreased levels of serum insulin like growth factor-1 (IGF-1) and of insulin. The properties of AS101 prompted us to investigate its potential therapeutic role in rats with type 2 diabetes (T2D). T2D was induced by a high fat diet combined with a low dose of Streptozotocin (STZ). Treatment with AS101 before manifestation of hyperglycemia, resulted in increased insulin sensitivity, and decreased blood glucose levels, and prevented symptoms of diabetes including defective glucose clearance, fatty liver, and abnormal distribution of insulin-producing beta cells in the pancreas. Treatment after disease emergence resulted in partial restoration of normal glucose homeostasis. Diabetic rats showed a reduction in liver SIRT1 levels. In both treatment regimens the reduction in SIRT1 levels in the liver were blocked by AS101 consumption. Together, these findings demonstrate the therapeutic potential of AS101 for treating T2D, and for reversing impaired fat and glucose metabolism.
Conflict of interest statement
The authors of this manuscript have no conflict of interest to declare.
Figures

References
-
- Zierath JR, He L, Guma A, Odegoard Wahlstrom E, Klip A, Wallberg-Henriksson H. Insulin action on glucose transport and plasma membrane GLUT4 content in skeletal muscle from patients with NIDDM. Diabetologia. 1996;39:1180–1189. - PubMed
-
- Kahn CR, Vicent D, Doria A. Genetics of non-insulin-dependent (type-II) diabetes mellitus. Annu Rev Med. 1996;47:509–531. - PubMed
-
- Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–787. - PubMed
-
- Saltiel AR. New perspectives into the molecular pathogenesis and treatment of type 2 diabetes. Cell. 2001;104:517–529. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

