Effects of proinflammatory cytokines on the claudin-19 rich tight junctions of human retinal pigment epithelium
- PMID: 22761260
- PMCID: PMC3410691
- DOI: 10.1167/iovs.11-8311
Effects of proinflammatory cytokines on the claudin-19 rich tight junctions of human retinal pigment epithelium
Abstract
Purpose: Chronic, subclinical inflammation contributes to the pathogenesis of several ocular diseases, including age-related macular degeneration. Proinflammatory cytokines affect tight junctions in epithelia that lack claudin-19, but in the retinal pigment epithelium claudin-19 predominates. We examined the effects of cytokines on the tight junctions of human fetal RPE (hfRPE).
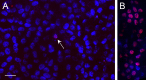
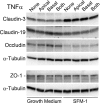
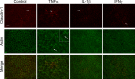
Methods: hfRPE was incubated with interleukin 1-beta (IL-1β), interferon-gamma (IFNγ), or tumor necrosis factor-alpha (TNFα), alone or in combination. Permeability and selectivity of the tight junctions were assessed using nonionic tracers and electrophysiology. Claudins, occludin, and ZO-1 were examined using PCR, immunoblotting, and confocal immunofluorescence microscopy.
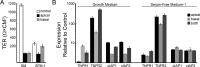
Results: Only TNFα consistently reduced transepithelial electrical resistance (TER) >80%. A serum-free medium revealed two effects of TNFα: (1) decreased TER was observed only when TNFα was added to the apical side of the monolayer, and (2) expression of TNFα receptors and inhibitors of apoptosis were induced from either side of the monolayer. In untreated cultures, tight junctions were slightly cation selective, and this was affected minimally by TNFα. The results were unexplained by effects on claudin-2, claudin-3, claudin-19, occludin, and ZO-1, but changes in the morphology of the junctions and actin cytoskeleton may have a role.
Conclusions: Claudin-19-rich tight junctions have low permeability for ionic and nonionic solutes, and are slightly cation-selective. Claudin-19 is not a direct target of TNFα. TNFα may protect RPE from apoptosis, but makes the monolayer leaky when it is presented to the apical side of the monolayer. Unlike other epithelia, IFNγ failed to augment the effect of TNFα on tight junctions.
Conflict of interest statement
Disclosure:
Figures




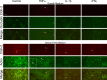
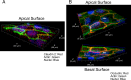



Similar articles
-
Claudin-19 and the barrier properties of the human retinal pigment epithelium.Invest Ophthalmol Vis Sci. 2011 Mar 14;52(3):1392-403. doi: 10.1167/iovs.10-5984. Print 2011 Mar. Invest Ophthalmol Vis Sci. 2011. PMID: 21071746 Free PMC article.
-
Minimal effects of VEGF and anti-VEGF drugs on the permeability or selectivity of RPE tight junctions.Invest Ophthalmol Vis Sci. 2010 Jun;51(6):3216-25. doi: 10.1167/iovs.09-4162. Epub 2009 Dec 30. Invest Ophthalmol Vis Sci. 2010. PMID: 20042644 Free PMC article.
-
Proinflammatory cytokines tumor necrosis factor-alpha and interferon-gamma modulate epithelial barrier function in Madin-Darby canine kidney cells through mitogen activated protein kinase signaling.BMC Physiol. 2006 Feb 21;6:2. doi: 10.1186/1472-6793-6-2. BMC Physiol. 2006. PMID: 16504032 Free PMC article.
-
Ruffles and spikes: Control of tight junction morphology and permeability by claudins.Biochim Biophys Acta Biomembr. 2020 Sep 1;1862(9):183339. doi: 10.1016/j.bbamem.2020.183339. Epub 2020 May 7. Biochim Biophys Acta Biomembr. 2020. PMID: 32389670 Free PMC article. Review.
-
Integration of tight junctions and claudins with the barrier functions of the retinal pigment epithelium.Prog Retin Eye Res. 2011 Sep;30(5):296-323. doi: 10.1016/j.preteyeres.2011.06.002. Epub 2011 Jun 17. Prog Retin Eye Res. 2011. PMID: 21704180 Review.
Cited by
-
Basolateral activation with TLR agonists induces polarized cytokine release and reduces barrier function in RPE in vitro.Graefes Arch Clin Exp Ophthalmol. 2021 Feb;259(2):413-424. doi: 10.1007/s00417-020-04930-2. Epub 2020 Sep 19. Graefes Arch Clin Exp Ophthalmol. 2021. PMID: 32949301 Free PMC article.
-
Complement C3 Activation in the Human Retinal Pigment Epithelium.Invest Ophthalmol Vis Sci. 2025 Jul 1;66(9):67. doi: 10.1167/iovs.66.9.67. Invest Ophthalmol Vis Sci. 2025. PMID: 40728360 Free PMC article.
-
Engineering a blood-retinal barrier with human embryonic stem cell-derived retinal pigment epithelium: transcriptome and functional analysis.Stem Cells Transl Med. 2013 Jul;2(7):534-44. doi: 10.5966/sctm.2012-0134. Epub 2013 Jun 3. Stem Cells Transl Med. 2013. PMID: 23734062 Free PMC article.
-
NLRP3 Inflammasome and Pathobiology in AMD.J Clin Med. 2015 Jan 14;4(1):172-92. doi: 10.3390/jcm4010172. J Clin Med. 2015. PMID: 26237026 Free PMC article. Review.
-
Targeting Neovascularization in Ischemic Retinopathy: Recent Advances.Expert Rev Ophthalmol. 2013 Jun;8(3):267-286. doi: 10.1586/eop.13.17. Expert Rev Ophthalmol. 2013. PMID: 25598837 Free PMC article.
References
-
- Demircan N, Safran BG, Soylu M, Ozcan AA, Sizmaz S. Determination of vitreous interleukin-1 (IL-1) and tumour necrosis factor (TNF) levels in proliferative diabetic retinopathy. Eye (Lond). 2006;20:1366–1369 - PubMed
-
- Foxman EF, Zhang M, Hurst SD, et al. Inflammatory mediators in uveitis: differential induction of cytokines and chemokines in Th1- versus Th2-mediated ocular inflammation. J Immunol. 2002;168:2483–2492 - PubMed
-
- Takase H, Futagami Y, Yoshida T, et al. Cytokine profile in aqueous humor and sera of patients with infectious or noninfectious uveitis. Invest Ophthalmol Vis Sci. 2006;47:1557–1561 - PubMed
-
- Cousins SW, Espinosa-Heidmann DG, Csaky KG. Monocyte activation in patients with age-related macular degeneration: a biomarker of risk for choroidal neovascularization? Arch Ophthalmol. 2004;122:1013–1018 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

