Duplicated zebrafish co-orthologs of parathyroid hormone-related peptide (PTHrP, Pthlh) play different roles in craniofacial skeletogenesis
- PMID: 22761277
- PMCID: PMC3718479
- DOI: 10.1530/JOE-12-0110
Duplicated zebrafish co-orthologs of parathyroid hormone-related peptide (PTHrP, Pthlh) play different roles in craniofacial skeletogenesis
Abstract
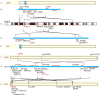
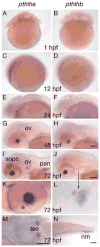
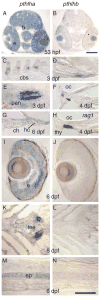
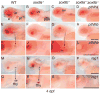
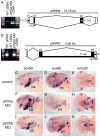
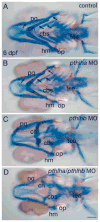
In mammals, parathyroid hormone-related peptide (PTHrP, alias PTH-like hormone (Pthlh)) acts as a paracrine hormone that regulates the patterning of cartilage, bone, teeth, pancreas, and thymus. Beyond mammals, however, little is known about the molecular genetic mechanisms by which Pthlh regulates early development. To evaluate conserved pathways of craniofacial skeletogenesis, we isolated two Pthlh co-orthologs from the zebrafish (Danio rerio) and investigated their structural, phylogenetic, and syntenic relationships, expression, and function. Results showed that pthlh duplicates originated in the teleost genome duplication. Zebrafish pthlha and pthlhb were maternally expressed and showed overlapping and distinct zygotic expression patterns during skeletal development that mirrored mammalian expression domains. To explore the regulation of duplicated pthlh genes, we studied their expression patterns in mutants and found that both sox9a and sox9b are upstream of pthlha in arch and fin bud cartilages, but only sox9b is upstream of pthlha in the pancreas. Morpholino antisense knockdown showed that pthlha regulates both sox9a and sox9b in the pharyngeal arches but not in the brain or otic vesicles and that pthlhb does not regulate either sox9 gene, which is likely related to its highly degraded nuclear localization signal. Knockdown of pthlha but not pthlhb caused runx2b overexpression in craniofacial cartilages and premature bone mineralization. We conclude that in normal cartilage development, sox9 upregulates pthlh, which downregulates runx2, and that the duplicated nature of all three of these genes in zebrafish creates a network of regulation by different co-orthologs in different tissues.
Conflict of interest statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

