Controlled inflammatory responses in the lungs are associated with protection elicited by a pneumococcal surface protein A-based vaccine against a lethal respiratory challenge with Streptococcus pneumoniae in mice
- PMID: 22761301
- PMCID: PMC3428385
- DOI: 10.1128/CVI.00171-12
Controlled inflammatory responses in the lungs are associated with protection elicited by a pneumococcal surface protein A-based vaccine against a lethal respiratory challenge with Streptococcus pneumoniae in mice
Abstract
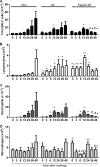
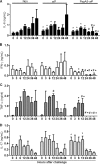
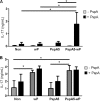
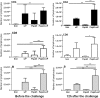
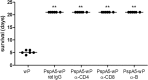
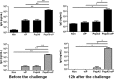
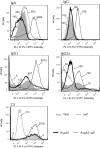
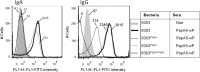
Streptococcus pneumoniae is a pathogen of great importance worldwide. We have previously described the efficacy of a nasal vaccine composed of the pneumococcal surface protein A and the whole-cell pertussis vaccine as an adjuvant against a pneumococcal invasive challenge in mice. Spread of bacteria to the bloodstream was probably prevented by the high levels of systemic antibodies induced by the vaccine, but bacteria were only cleared from the lungs 3 weeks later, indicating that local immune responses may contribute to survival. Here we show that a strict control of inflammatory responses in lungs of vaccinated mice occurs even in the presence of high numbers of pneumococci. This response was characterized by a sharp peak of neutrophils and lymphocytes with a simultaneous decrease in macrophages in the respiratory mucosa at 12 h postchallenge. Secretion of interleukin-6 (IL-6) and gamma interferon (IFN-γ) was reduced at 24 h postchallenge, and the induction of tumor necrosis factor alpha (TNF-α) secretion, observed in the first hours postchallenge, was completely abolished at 24 h. Before challenge and at 12 h postchallenge, vaccinated mice displayed higher numbers of CD4(+) T, CD8(+) T, and B lymphocytes in the lungs. However, protection still occurs in the absence of each of these cells during the challenge, indicating that other effectors may be related to the prevention of lung injuries in this model. High levels of mucosal anti-PspA antibodies were maintained in vaccinated mice during the challenge, suggesting an important role in protection.
Figures

Similar articles
-
Protection elicited by nasal immunization with pneumococcal surface protein A (PspA) adjuvanted with bacterium-like particles against Streptococcus pneumoniae infection in mice.Microb Pathog. 2018 Oct;123:115-119. doi: 10.1016/j.micpath.2018.06.041. Epub 2018 Jun 26. Microb Pathog. 2018. PMID: 29959043
-
Characterization of protective mucosal and systemic immune responses elicited by pneumococcal surface protein PspA and PspC nasal vaccines against a respiratory pneumococcal challenge in mice.Clin Vaccine Immunol. 2009 May;16(5):636-45. doi: 10.1128/CVI.00395-08. Epub 2009 Mar 11. Clin Vaccine Immunol. 2009. PMID: 19279169 Free PMC article.
-
Combination of pneumococcal surface protein A (PspA) with whole cell pertussis vaccine increases protection against pneumococcal challenge in mice.PLoS One. 2010 May 27;5(5):e10863. doi: 10.1371/journal.pone.0010863. PLoS One. 2010. PMID: 20523738 Free PMC article.
-
Pertussis toxin improves immune responses to a combined pneumococcal antigen and leads to enhanced protection against Streptococcus pneumoniae.Clin Vaccine Immunol. 2014 Jul;21(7):972-81. doi: 10.1128/CVI.00134-14. Epub 2014 May 7. Clin Vaccine Immunol. 2014. PMID: 24807055 Free PMC article.
-
Dendritic cell-targeting DNA-based nasal adjuvants for protective mucosal immunity to Streptococcus pneumoniae.Microbiol Immunol. 2017 Jun;61(6):195-205. doi: 10.1111/1348-0421.12487. Microbiol Immunol. 2017. PMID: 28463465 Free PMC article. Review.
Cited by
-
Early pneumococcal clearance in mice induced by systemic immunization with recombinant BCG PspA-PdT prime and protein boost correlates with cellular and humoral immune response in bronchoalveolar fluids (BALF).Vaccine X. 2019 Dec 3;4:100049. doi: 10.1016/j.jvacx.2019.100049. eCollection 2020 Apr 9. Vaccine X. 2019. PMID: 31891153 Free PMC article.
-
PilVax - a novel peptide delivery platform for the development of mucosal vaccines.Sci Rep. 2018 Feb 7;8(1):2555. doi: 10.1038/s41598-018-20863-7. Sci Rep. 2018. PMID: 29416095 Free PMC article.
-
Protection Elicited by Nasal Immunization with Recombinant Pneumococcal Surface Protein A (rPspA) Adjuvanted with Whole-Cell Pertussis Vaccine (wP) against Co-Colonization of Mice with Streptococcus pneumoniae.PLoS One. 2017 Jan 19;12(1):e0170157. doi: 10.1371/journal.pone.0170157. eCollection 2017. PLoS One. 2017. PMID: 28103277 Free PMC article.
-
Robust Immune Response and Protection against Lethal Pneumococcal Challenge with a Recombinant BCG-PspA-PdT Prime/Boost Scheme Administered to Neonatal Mice.Vaccines (Basel). 2024 Jan 25;12(2):122. doi: 10.3390/vaccines12020122. Vaccines (Basel). 2024. PMID: 38400107 Free PMC article.
-
Impaired expression of CXCL5 and matrix metalloproteinases in the lungs of mice with high susceptibility to Streptococcus pneumoniae infection.Immun Inflamm Dis. 2018 Mar;6(1):128-142. doi: 10.1002/iid3.205. Epub 2017 Nov 9. Immun Inflamm Dis. 2018. PMID: 29119707 Free PMC article.
References
-
- Achen MG, Davidson BE, Hillier AJ. 1986. Construction of plasmid vectors for the detection of streptococcal promoters. Gene 45:45–49 - PubMed
-
- Aida Y, Pabst MJ. 1990. Removal of endotoxin from protein solutions by phase separation using Triton X-114. J. Immunol. Methods 132:191–195 - PubMed
-
- Andreasen C, Powell DA, Carbonetti NH. 2009. Pertussis toxin stimulates IL-17 production in response to Bordetella pertussis infection in mice. PLoS One 4:e7079 doi:10.1371/journal.pone.0007079 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

