A soluble bone morphogenetic protein type IA receptor increases bone mass and bone strength
- PMID: 22761317
- PMCID: PMC3409793
- DOI: 10.1073/pnas.1204929109
A soluble bone morphogenetic protein type IA receptor increases bone mass and bone strength
Abstract
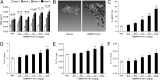
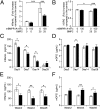
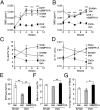
Diseases such as osteoporosis are associated with reduced bone mass. Therapies to prevent bone loss exist, but there are few that stimulate bone formation and restore bone mass. Bone morphogenetic proteins (BMPs) are members of the TGFβ superfamily, which act as pleiotropic regulators of skeletal organogenesis and bone homeostasis. Ablation of the BMPR1A receptor in osteoblasts increases bone mass, suggesting that inhibition of BMPR1A signaling may have therapeutic benefit. The aim of this study was to determine the skeletal effects of systemic administration of a soluble BMPR1A fusion protein (mBMPR1A-mFc) in vivo. mBMPR1A-mFc was shown to bind BMP2/4 specifically and with high affinity and prevent downstream signaling. mBMPR1A-mFc treatment of immature and mature mice increased bone mineral density, cortical thickness, trabecular bone volume, thickness and number, and decreased trabecular separation. The increase in bone mass was due to an early increase in osteoblast number and bone formation rate, mediated by a suppression of Dickkopf-1 expression. This was followed by a decrease in osteoclast number and eroded surface, which was associated with a decrease in receptor activator of NF-κB ligand (RANKL) production, an increase in osteoprotegerin expression, and a decrease in serum tartrate-resistant acid phosphatase (TRAP5b) concentration. mBMPR1A treatment also increased bone mass and strength in mice with bone loss due to estrogen deficiency. In conclusion, mBMPR1A-mFc stimulates osteoblastic bone formation and decreases bone resorption, which leads to an increase in bone mass, and offers a promising unique alternative for the treatment of bone-related disorders.
Conflict of interest statement
Conflict of interest statement: N.S., M.C.-B., D.S., Y.K., K.L., K.W.U., J.U., R.K., E.P., A.G., J.S., R.S.P. are employees of Acceleron Pharma. P.I.C., M.L.B., and E.C. have received research funding from Acceleron Pharma.
Figures




References
-
- Wozney JM. Bone morphogenetic proteins. Prog Growth Factor Res. 1989;1:267–280. - PubMed
-
- Massagué J. Receptors for the TGF-beta family. Cell. 1992;69:1067–1070. - PubMed
-
- Keller S, Nickel J, Zhang JL, Sebald W, Mueller TD. Molecular recognition of BMP-2 and BMP receptor IA. Nat Struct Mol Biol. 2004;11:481–488. - PubMed
-
- Hatta T, et al. Identification of the ligand-binding site of the BMP type IA receptor for BMP-4. Biopolymers. 2000;55:399–406. - PubMed
-
- Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22:233–241. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

