Interferon response factors 3 and 7 protect against Chikungunya virus hemorrhagic fever and shock
- PMID: 22761364
- PMCID: PMC3446587
- DOI: 10.1128/JVI.00956-12
Interferon response factors 3 and 7 protect against Chikungunya virus hemorrhagic fever and shock
Abstract
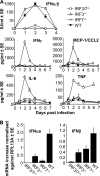
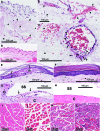
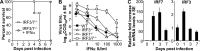
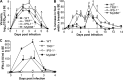
Chikungunya virus (CHIKV) infections can produce severe disease and mortality. Here we show that CHIKV infection of adult mice deficient in interferon response factors 3 and 7 (IRF3/7(-/-)) is lethal. Mortality was associated with undetectable levels of alpha/beta interferon (IFN-α/β) in serum, ∼50- and ∼10-fold increases in levels of IFN-γ and tumor necrosis factor (TNF), respectively, increased virus replication, edema, vasculitis, hemorrhage, fever followed by hypothermia, oliguria, thrombocytopenia, and raised hematocrits. These features are consistent with hemorrhagic shock and were also evident in infected IFN-α/β receptor-deficient mice. In situ hybridization suggested CHIKV infection of endothelium, fibroblasts, skeletal muscle, mononuclear cells, chondrocytes, and keratinocytes in IRF3/7(-/-) mice; all but the latter two stained positive in wild-type mice. Vaccination protected IRF3/7(-/-) mice, suggesting that defective antibody responses were not responsible for mortality. IPS-1- and TRIF-dependent pathways were primarily responsible for IFN-α/β induction, with IRF7 being upregulated >100-fold in infected wild-type mice. These studies suggest that inadequate IFN-α/β responses following virus infection can be sufficient to induce hemorrhagic fever and shock, a finding with implications for understanding severe CHIKV disease and dengue hemorrhagic fever/dengue shock syndrome.
Figures


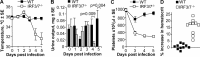
References
-
- Ali U, Isahak I, Rahman MM. 2011. Chikungunya confused with dengue In Malaysia: clinical, serological and molecular perspective. Internet J. Microbiol. 9(2). doi: - DOI
-
- Buss C, et al. 2010. Essential role of mitochondrial antiviral signaling, IFN-regulatory factor (IRF)3, and IRF7 in Chlamydophila pneumoniae-mediated IFN-beta response and control of bacterial replication in human endothelial cells. J. Immunol. 184:3072–3078 - PubMed
-
- Chen LH, Wilson ME. 2010. Dengue and chikungunya infections in travelers. Curr. Opin. Infect. Dis. 23:438–444 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

