Binding of cellular export factor REF/Aly by Kaposi's sarcoma-associated herpesvirus (KSHV) ORF57 protein is not required for efficient KSHV lytic replication
- PMID: 22761374
- PMCID: PMC3446620
- DOI: 10.1128/JVI.01190-12
Binding of cellular export factor REF/Aly by Kaposi's sarcoma-associated herpesvirus (KSHV) ORF57 protein is not required for efficient KSHV lytic replication
Abstract
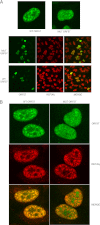
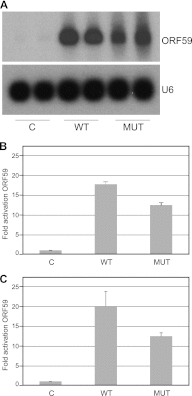
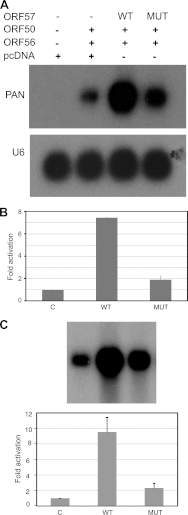
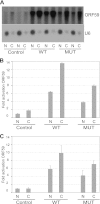
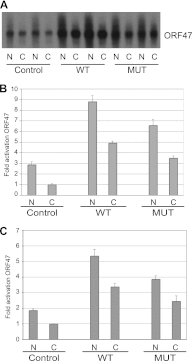
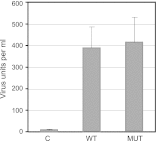
Kaposi's sarcoma-associated herpesvirus (KSHV) ORF57 protein is expressed early during lytic KSHV replication, enhances expression of many KSHV genes, and is essential for virus production. ORF57 is a member of a family of proteins conserved among all human and many animal herpesviruses that are multifunctional regulators of gene expression and act posttranscriptionally to increase accumulation of their target mRNAs. The mechanism of ORF57 action is complex and may involve effects on mRNA transcription, stability, and export. ORF57 directly binds to REF/Aly, a cellular RNA-binding protein component of the TREX complex that mediates RNA transcription and export. We analyzed the effects of an ORF57 mutation known to abrogate REF/Aly binding and demonstrate that the REF-binding mutant is impaired in activation of viral mRNAs and noncoding RNAs confined to the nucleus. Although the inability to bind REF leads to decreased ORF57 activity in enhancing gene expression, there is no demonstrable effect on nuclear export of viral mRNA or the ability of ORF57 to support KSHV replication and virus production. These data indicate that REF/Aly-ORF57 interaction is not essential for KSHV lytic replication but may contribute to target RNA stability independent of effects on RNA export, suggesting a novel role for REF/Aly in viral RNA metabolism.
Figures


References
-
- Bello LJ, et al. 1999. The human herpesvirus-8 ORF 57 gene and its properties. J. Gen. Virol. 80:3207–3215 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

