Alanine scanning of poliovirus 2CATPase reveals new genetic evidence that capsid protein/2CATPase interactions are essential for morphogenesis
- PMID: 22761387
- PMCID: PMC3446611
- DOI: 10.1128/JVI.00914-12
Alanine scanning of poliovirus 2CATPase reveals new genetic evidence that capsid protein/2CATPase interactions are essential for morphogenesis
Abstract
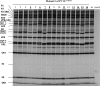
Polypeptide 2C(ATPase) is one of the most thoroughly studied but least understood proteins in the life cycle of poliovirus. Within the protein, multiple functional domains important for uncoating, host cell membrane alterations, and RNA replication and encapsidation have previously been identified. In this study, charged to alanine-scanning mutagenesis was used to generate conditional-lethal mutations in hitherto-uncharacterized domains of the 2C(ATPase) polypeptide, particularly those involved in morphogenesis. Adjacent or clustered charged amino acids (2 to 4), scattered along the 2C(ATPase) coding sequence, were replaced with alanines. RNA transcripts of mutant poliovirus cDNA clones were transfected into HeLa cells. Subsequently, 10 lethal, 1 severely temperature-sensitive, 2 quasi-infectious, and 3 wild type-like mutants were identified. Using a luciferase-containing reporter virus, we demonstrated RNA replication defects in all lethal and quasi-infectious mutants. Temperature-sensitive mutants were defective in RNA replication only at the restricted temperatures. Furthermore, we characterized a quasi-infectious mutant (K(6)A/K(7)A) that produced a suppressor mutation (G(1)R) and a novel 2B^2C(ATPase) cleavage site (Q^R). Surprisingly, this cleavage site mutation did not interfere with normal processing of the polyprotein. These mutants have led to the identification of several new sites within the 2C(ATPase) polypeptide that are required for RNA replication. In addition, analysis of the suppressor mutants has revealed a new domain near the C terminus of 2C(ATPase) that is involved in encapsidation, possibly achieved through interaction with an amino acid sequence between NTP binding motifs A and B of 2C(ATPase). Most importantly, the identification of suppressor mutations in both 2C(ATPase) and the capsid domains (VP1 and VP3) of poliovirus has confirmed that an interaction between 2C(ATPase) and capsid proteins is involved in viral morphogenesis.
Figures


References
-
- Aldabe R, Carrasco L. 1995. Induction of membrane proliferation by poliovirus proteins 2C and 2BC. Biochem. Biophys. Res. Commun. 206:64–76 - PubMed
-
- Baltimore D. 1969. Biochemistry of viruses. Marcel Dekker, Inc., New York, NY
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

