Polycyclic aromatic hydrocarbons (PAHs) mediate transcriptional activation of the ATP binding cassette transporter ABCB6 gene via the aryl hydrocarbon receptor (AhR)
- PMID: 22761424
- PMCID: PMC3442536
- DOI: 10.1074/jbc.M112.371476
Polycyclic aromatic hydrocarbons (PAHs) mediate transcriptional activation of the ATP binding cassette transporter ABCB6 gene via the aryl hydrocarbon receptor (AhR)
Abstract
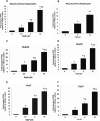
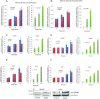
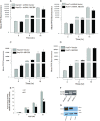
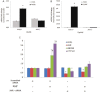
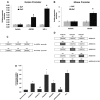
Liver is endowed with a mechanism to induce hepatic cytochromes P450 (CYP450s) in response to therapeutic drugs and environmental contaminants, leading to increased detoxification and elimination of the xenobiotics. Each CYP450 is composed of an apoprotein moiety and a heme prosthetic group, which is required for CYP450 activity. Thus, under conditions of CYP450 induction, there is a coordinate increase in heme biosynthesis to compensate for the increased expression of CYP450s. ABCB6, a mitochondrial ATP binding cassette transporter, which regulates coproporphyrinogen transport from the cytoplasm into the mitochondria to complete heme biosynthesis, represents a previously unrecognized rate-limiting step in heme biosynthesis. However, it is not known if exposure to drugs and environmental contaminants induces ABCB6 expression, to assure an adequate and apparently coordinated supply of heme for the generation of functional cytochrome holoprotein. In the present study, we demonstrate that polycyclic aromatic hydrocarbons (PAHs), the widely distributed environmental toxicants shown to induce porphyrin accumulation causing hepatic porphyria, up-regulate ABCB6 expression in both mice and humans. Using siRNA technology and Abcb6 knock-out mice, we demonstrate that PAH-mediated increase in hepatic porphyrins is compromised in the absence of ABCB6. Moreover, in vivo studies in aryl hydrocarbon receptor (AhR) knock-out mice demonstrate that PAH induction of ABCB6 is mediated by AhR. Promoter activation studies combined with electrophoretic mobility shift assay and chromatin immunoprecipitation assay demonstrate direct interactions between the AhR binding sites in the ABCB6 promoter and the AhR receptor, implicating drug activation mechanisms for ABCB6 similar to those found in inducible cytochrome P450s. These studies are the first to describe direct transcriptional activation of both mouse and human ABCB6 by xenobiotics.
Figures



Similar articles
-
Polycyclic Aromatic Hydrocarbons Activate the Aryl Hydrocarbon Receptor and the Constitutive Androstane Receptor to Regulate Xenobiotic Metabolism in Human Liver Cells.Int J Mol Sci. 2020 Dec 31;22(1):372. doi: 10.3390/ijms22010372. Int J Mol Sci. 2020. PMID: 33396476 Free PMC article.
-
ATP-dependent mitochondrial porphyrin importer ABCB6 protects against phenylhydrazine toxicity.J Biol Chem. 2012 Apr 13;287(16):12679-90. doi: 10.1074/jbc.M111.336180. Epub 2012 Jan 31. J Biol Chem. 2012. PMID: 22294697 Free PMC article.
-
Polycyclic aromatic hydrocarbon-inducible DNA adducts: evidence by 32P-postlabeling and use of knockout mice for Ah receptor-independent mechanisms of metabolic activation in vivo.Int J Cancer. 2003 Jan 1;103(1):5-11. doi: 10.1002/ijc.10784. Int J Cancer. 2003. PMID: 12455047
-
Role of coactivators in transcriptional activation by the aryl hydrocarbon receptor.Arch Biochem Biophys. 2005 Jan 15;433(2):379-86. doi: 10.1016/j.abb.2004.09.031. Arch Biochem Biophys. 2005. PMID: 15581594 Review.
-
Immunotoxicity of Xenobiotics in Fish: A Role for the Aryl Hydrocarbon Receptor (AhR)?Int J Mol Sci. 2021 Aug 31;22(17):9460. doi: 10.3390/ijms22179460. Int J Mol Sci. 2021. PMID: 34502366 Free PMC article. Review.
Cited by
-
Role of neurotoxicants in the pathogenesis of Alzheimer's disease: a mechanistic insight.Ann Med. 2021 Dec;53(1):1476-1501. doi: 10.1080/07853890.2021.1966088. Ann Med. 2021. PMID: 34433343 Free PMC article. Review.
-
Differences in smoking associated DNA methylation patterns in South Asians and Europeans.Clin Epigenetics. 2014 Feb 3;6(1):4. doi: 10.1186/1868-7083-6-4. Clin Epigenetics. 2014. PMID: 24485148 Free PMC article.
-
Efficient purification and reconstitution of ATP binding cassette transporter B6 (ABCB6) for functional and structural studies.J Biol Chem. 2013 Aug 2;288(31):22658-69. doi: 10.1074/jbc.M113.485284. Epub 2013 Jun 21. J Biol Chem. 2013. PMID: 23792964 Free PMC article.
-
Benzo(a)pyrene and cardiovascular diseases: An overview of pre-clinical studies focused on the underlying molecular mechanism.Front Nutr. 2022 Aug 4;9:978475. doi: 10.3389/fnut.2022.978475. eCollection 2022. Front Nutr. 2022. PMID: 35990352 Free PMC article. Review.
-
Dormant cancer cells accumulate high protoporphyrin IX levels and are sensitive to 5-aminolevulinic acid-based photodynamic therapy.Sci Rep. 2016 Nov 18;6:36478. doi: 10.1038/srep36478. Sci Rep. 2016. PMID: 27857072 Free PMC article.
References
-
- Ponka P. (1999) Cell biology of heme. Am. J. Med. Sci. 318, 241–256 - PubMed
-
- Padmanaban G., Venkateswar V., Rangarajan P. N. (1989) Heme as a multifunctional regulator. Trends Biochem. Sci. 14, 492–496 - PubMed
-
- Sassa S., Nagai T. (1996) The role of heme in gene expression. Int. J. Hematol. 63, 167–178 - PubMed
-
- Aft R. L., Mueller G. C. (1984) Hemin-mediated oxidative degradation of proteins. J. Biol. Chem. 259, 301–305 - PubMed
-
- Maines M. D., Kappas A. (1975) The degradative effects of porphyrins and heme compounds on components of the microsomal mixed function oxidase system. J. Biol. Chem. 250, 2363–2369 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

