Long isoform mouse selenoprotein P (Sepp1) supplies rat myoblast L8 cells with selenium via endocytosis mediated by heparin binding properties and apolipoprotein E receptor-2 (ApoER2)
- PMID: 22761431
- PMCID: PMC3436540
- DOI: 10.1074/jbc.M112.383521
Long isoform mouse selenoprotein P (Sepp1) supplies rat myoblast L8 cells with selenium via endocytosis mediated by heparin binding properties and apolipoprotein E receptor-2 (ApoER2)
Abstract
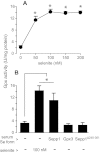
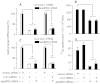
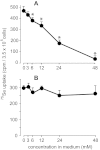
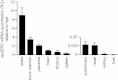
In vivo studies have shown that selenium is supplied to testis and brain by apoER2-mediated endocytosis of Sepp1. Although cultured cell lines have been shown to utilize selenium from Sepp1 added to the medium, the mechanism of uptake and utilization has not been characterized. Rat L8 myoblast cells were studied. They took up mouse Sepp1 from the medium and used its selenium to increase their glutathione peroxidase (Gpx) activity. L8 cells did not utilize selenium from Gpx3, the other plasma selenoprotein. Neither did they utilize it from Sepp1(Δ240-361), the isoform of Sepp1 that lacks the selenium-rich C-terminal domain. To identify Sepp1 receptors, a solubilized membrane fraction was passed over a Sepp1 column. The receptors apoER2 and Lrp1 were identified in the eluate by mass spectrometry. siRNA experiments showed that knockdown of apoER2, but not of Lrp1, inhibited (75)Se uptake from (75)Se-labeled Sepp1. The addition of protamine to the medium or treatment of the cells with chlorate also inhibited (75)Se uptake. Blockage of lysosome acidification did not inhibit uptake of Sepp1 but did prevent its digestion and thereby utilization of its selenium. These results indicate that L8 cells take up Sepp1 by an apoER2-mediated mechanism requiring binding to heparin sulfate proteoglycans. The presence of at least part of the selenium-rich C-terminal domain of Sepp1 is required for uptake. RT-PCR showed that mouse tissues express apoER2 in varying amounts. It is postulated that apoER2-mediated uptake of long isoform Sepp1 is responsible for selenium distribution to tissues throughout the body.
Figures



References
-
- Hill K. E., Zhou J., McMahan W. J., Motley A. K., Atkins J. F., Gesteland R. F., Burk R. F. (2003) Deletion of selenoprotein P alters distribution of selenium in the mouse. J. Biol. Chem. 278, 13640–13646 - PubMed
-
- Burk R. F., Hill K. E. (2005) Selenoprotein P. An extracellular protein with unique physical characteristics and a role in selenium homeostasis. Annu. Rev. Nutr. 25, 215–235 - PubMed
-
- Ma S., Hill K. E., Caprioli R. M., Burk R. F. (2002) Mass spectrometric characterization of full-length rat selenoprotein P and three isoforms shortened at the C terminus. Evidence that three UGA codons in the mRNA open reading frame have alternative functions of specifying selenocysteine insertion or translation termination. J. Biol. Chem. 277, 12749–12754 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

