Polyphosphate is a novel pro-inflammatory regulator of mast cells and is located in acidocalcisomes
- PMID: 22761438
- PMCID: PMC3436523
- DOI: 10.1074/jbc.M112.385823
Polyphosphate is a novel pro-inflammatory regulator of mast cells and is located in acidocalcisomes
Abstract
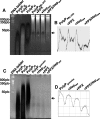
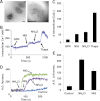
Polyphosphate (polyP) is a pro-inflammatory agent and a potent modulator of the human blood-clotting system. The presence of polyP of 60 phosphate units was identified in rat basophilic leukemia (RBL-2H3) mast cells using specific enzymatic assays, urea-polyacrylamide gel electrophoresis of cell extracts, and staining of cells with 4,6-diamidino-2-phenylindole (DAPI), and the polyP-binding domain of Escherichia coli exopolyphosphatase. PolyP co-localizes with serotonin- but not with histamine-containing granules. PolyP levels greatly decreased in mast cells stimulated to degranulate by IgE. Mast cell granules were isolated and found to be acidic and decrease their polyP content upon alkalinization. In agreement with these results, when RBL-2H3 mast cells were loaded with the fluorescent calcium indicator fura-2 acetoxymethyl ester to measure their intracellular Ca(2+) concentration ([Ca(2+)](i)), they were shown to possess a significant amount of Ca(2+) stored in an acidic compartment different from lysosomes. PolyP derived from RBL-2H3 mast cells stimulated bradykinin formation, and it was also detected in human basophils. All of these characteristics of mast cell granules, together with their known elemental composition, and high density, are similar to those of acidocalcisomes. The results suggest that mast cells polyP could be an important mediator of their pro-inflammatory and pro-coagulant activities.
Figures





References
-
- Rao N. N., Gómez-García M. R., Kornberg A. (2009) Inorganic polyphosphate: essential for growth and survival. Annu. Rev. Biochem. 78, 605–647 - PubMed
-
- Ruiz F. A., Lea C. R., Oldfield E., Docampo R. (2004) Human platelet dense granules contain polyphosphate and are similar to acidocalcisomes of bacteria and unicellular eukaryotes. J. Biol. Chem. 279, 44250–44257 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

