Identification of the actin and plasminogen binding regions of group B streptococcal phosphoglycerate kinase
- PMID: 22761440
- PMCID: PMC3436549
- DOI: 10.1074/jbc.M112.361261
Identification of the actin and plasminogen binding regions of group B streptococcal phosphoglycerate kinase
Abstract
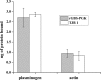
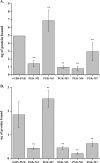
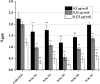
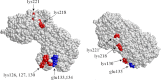
Phosphoglycerate kinase (PGK), present on the surface of group B streptococcus (GBS), has previously been demonstrated to bind the host proteins actin and plasminogen. The actin and plasminogen binding sites of GBS-PGK were identified using truncated GBS-PGK molecules, followed by peptide mapping. These experiments identified two actin and plasminogen binding sites located between amino acids 126-134 and 204-208 of the 398-amino acid-long GBS-PGK molecule. Substitution of the lysine residues within these regions with alanine resulted in significantly reduced binding to both actin and plasminogen. In addition, conversion of the glutamic acid residue at amino acid 133 to proline, the amino acid found at this position for the PGK protein of Streptococcus pneumoniae, also resulted in significantly reduced binding to actin and plasminogen. These results demonstrate that the lysine residues at amino acid positions 126, 127, 130, 204, and 208 along with the glutamic acid residue at amino acid position 133 are necessary for actin and plasminogen binding by GBS-PGK.
Figures


References
-
- Schuchat A., Robinson K., Wenger J. D., Harrison L. H., Farley M., Reingold A. L., Lefkowitz L., Perkins B. A. (1997) Bacterial meningitis in the United States in 1995. Active surveillance team. N. Engl. J. Med. 337, 970–976 - PubMed
-
- Weston E. J., Pondo T., Lewis M. M., Martell-Cleary P., Morin C., Jewell B., Daily P., Apostol M., Petit S., Farley M., Lynfield R., Reingold A., Hansen N. I., Stoll B. J., Shane A. J., Zell E., Schrag S. J. (2011) The burden of invasive early-onset neonatal sepsis in the United States, 2005–2008. Pediatr. Infect. Dis. J. 30, 937–941 - PMC - PubMed
-
- Edwards M. S., Baker C. J. (2005) Group B streptococcal infections in elderly adults. Clin. Infect. Dis. 41, 839–847 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

