Combined transfection of the three transcriptional factors, PDX-1, NeuroD1, and MafA, causes differentiation of bone marrow mesenchymal stem cells into insulin-producing cells
- PMID: 22761608
- PMCID: PMC3385644
- DOI: 10.1155/2012/672013
Combined transfection of the three transcriptional factors, PDX-1, NeuroD1, and MafA, causes differentiation of bone marrow mesenchymal stem cells into insulin-producing cells
Abstract
Aims: The goal of cell transcription for treatment of diabetes is to generate surrogate β-cells from an appropriate cell line. However, the induced replacement cells have showed less physiological function in producing insulin compared with normal β-cells.
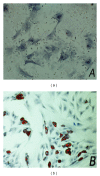
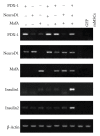
Methods: Here, we report a procedure for induction of insulin-producing cells (IPCs) from bone marrow murine mesenchymal stem cells (BM-mMSCs). These BM-mMSCs have the potential to differentiate into insulin-producing cells when a combination of PDX-1 (pancreatic and duodenal homeobox-1), NeuroD1 (neurogenic differentiation-1), and MafA (V-maf musculoaponeurotic fibrosarcoma oncogene homolog A) genes are transfected into them and expressed in these cells.
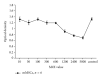
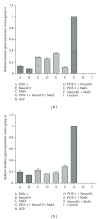
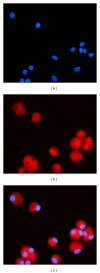
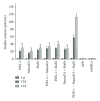
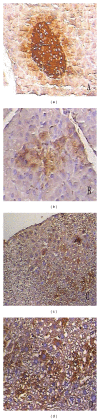
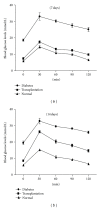
Results: Insulin biosynthesis and secretion were induced in mMSCs into which these three genes have been transfected and expressed. The amount of induced insulin in the mMSCs which have been transfected with the three genes together is significantly higher than in those mMSCs that were only transfected with one or two of these three genes. Transplantation of the transfected cells into mice with streptozotocin-induced diabetes results in insulin expression and the reversal of the glucose challenge.
Conclusions: These findings suggest major implications for cell replacement strategies in generation of surrogate β-cells for the treatment of diabetes.
Figures

References
-
- Best M, Carroll M, Hanley NA, Piper Hanley K. Embryonic stem cells to β-cells by understanding pancreas development. Molecular and Cellular Endocrinology. 2008;288(1-2):86–94. - PubMed
-
- Kim JH, Shin KH, Li TZ, Suh H. Potential of nucleofected human MSCs for insulin secretion. Journal of Tissue Engineering and Regenerative Medicine. 2011;5(10):761–769. - PubMed
-
- Li G, Luo R, Zhang J, et al. Generating mESC-derived insulin-producing cell lines through an intermediate lineage-restricted progenitor line. Stem Cell Research. 2009;2(1):41–55. - PubMed
-
- Naujok O, Francini F, Picton S, Bailey CJ, Lenzen S, Jörns A. Changes in gene expression and morphology of mouse embryonic stem cells on differentiation into insulin-producing cells in vitro and in vivo. Diabetes/Metabolism Research and Reviews. 2009;25(5):464–476. - PubMed
-
- Bernardo AS, Hay CW, Docherty K. Pancreatic transcription factors and their role in the birth, life and survival of the pancreatic β cell. Molecular and Cellular Endocrinology. 2008;294(1-2):1–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
