The safe use of a PTEN inhibitor for the activation of dormant mouse primordial follicles and generation of fertilizable eggs
- PMID: 22761722
- PMCID: PMC3384593
- DOI: 10.1371/journal.pone.0039034
The safe use of a PTEN inhibitor for the activation of dormant mouse primordial follicles and generation of fertilizable eggs
Abstract
Background: Primordial ovarian follicles, which are often present in the ovaries of premature ovarian failure (POF) patients or are cryopreserved from the ovaries of young cancer patients who are undergoing gonadotoxic anticancer therapies, cannot be used to generate mature oocytes for in vitro fertilization (IVF). There has been very little success in triggering growth of primordial follicles to obtain fertilizable oocytes due to the poor understanding of the biology of primordial follicle activation.
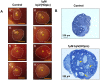
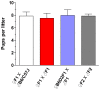
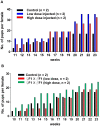
Methodology/principal findings: We have recently reported that PTEN (phosphatase and tensin homolog deleted on chromosome ten) prevents primordial follicle activation in mice, and deletion of Pten from the oocytes of primordial follicles leads to follicular activation. Consequently, the PTEN inhibitor has been successfully used in vitro to activate primordial follicles in both mouse and human ovaries. These results suggest that PTEN inhibitors could be used in ovarian culture medium to trigger the activation of primordial follicle. To study the safety and efficacy of the use of such inhibitors, we activated primordial follicles from neonatal mouse ovaries by transient treatment with a PTEN inhibitor bpV(HOpic). These ovaries were then transplanted under the kidney capsules of recipient mice to generate mature oocytes. The mature oocytes were fertilized in vitro and progeny mice were obtained after embryo transfer.
Results and conclusions: Long-term monitoring up to the second generation of progeny mice showed that the mice were reproductively active and were free from any overt signs or symptoms of chronic illnesses. Our results indicate that the use of PTEN inhibitors could be a safe and effective way of generating mature human oocytes for use in novel IVF techniques.
Conflict of interest statement
Figures

References
-
- Hirshfield AN. Development of follicles in the mammalian ovary. Int Rev Cytol. 1991;124:43–101. - PubMed
-
- McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;21:200–214. - PubMed
-
- Adhikari D, Liu K. Molecular mechanisms underlying the activation of mammalian primordial follicles. Endocr Rev. 2009;30:438–464. - PubMed
-
- Reddy P, Zheng W, Liu K. Mechanisms maintaining the dormancy and survival of mammalian primordial follicles. Trends Endocrinol Metab. 2010;21:96–103. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

