Cooperativity among short amyloid stretches in long amyloidogenic sequences
- PMID: 22761773
- PMCID: PMC3382238
- DOI: 10.1371/journal.pone.0039369
Cooperativity among short amyloid stretches in long amyloidogenic sequences
Abstract
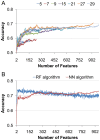
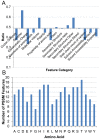
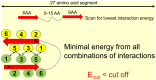
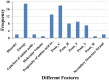
Amyloid fibrillar aggregates of polypeptides are associated with many neurodegenerative diseases. Short peptide segments in protein sequences may trigger aggregation. Identifying these stretches and examining their behavior in longer protein segments is critical for understanding these diseases and obtaining potential therapies. In this study, we combined machine learning and structure-based energy evaluation to examine and predict amyloidogenic segments. Our feature selection method discovered that windows consisting of long amino acid segments of ~30 residues, instead of the commonly used short hexapeptides, provided the highest accuracy. Weighted contributions of an amino acid at each position in a 27 residue window revealed three cooperative regions of short stretch, resemble the β-strand-turn-β-strand motif in A-βpeptide amyloid and β-solenoid structure of HET-s(218-289) prion (C). Using an in-house energy evaluation algorithm, the interaction energy between two short stretches in long segment is computed and incorporated as an additional feature. The algorithm successfully predicted and classified amyloid segments with an overall accuracy of 75%. Our study revealed that genome-wide amyloid segments are not only dependent on short high propensity stretches, but also on nearby residues.
Conflict of interest statement
Figures

References
-
- Trojanowski JQ, Mattson MP. Overview of protein aggregation in single, double, and triple neurodegenerative brain amyloidoses. Neuromolecular Med. 2003;4:1–6. - PubMed
-
- Dobson CM. Getting out of shape. Nature. 2002;418:729–730. - PubMed
-
- Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–366. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

