Simulation vs. reality: a comparison of in silico distance predictions with DEER and FRET measurements
- PMID: 22761805
- PMCID: PMC3382601
- DOI: 10.1371/journal.pone.0039492
Simulation vs. reality: a comparison of in silico distance predictions with DEER and FRET measurements
Abstract
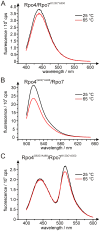
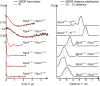
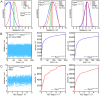
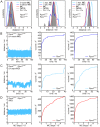
Site specific incorporation of molecular probes such as fluorescent- and nitroxide spin-labels into biomolecules, and subsequent analysis by Förster resonance energy transfer (FRET) and double electron-electron resonance (DEER) can elucidate the distance and distance-changes between the probes. However, the probes have an intrinsic conformational flexibility due to the linker by which they are conjugated to the biomolecule. This property minimizes the influence of the label side chain on the structure of the target molecule, but complicates the direct correlation of the experimental inter-label distances with the macromolecular structure or changes thereof. Simulation methods that account for the conformational flexibility and orientation of the probe(s) can be helpful in overcoming this problem. We performed distance measurements using FRET and DEER and explored different simulation techniques to predict inter-label distances using the Rpo4/7 stalk module of the M. jannaschii RNA polymerase. This is a suitable model system because it is rigid and a high-resolution X-ray structure is available. The conformations of the fluorescent labels and nitroxide spin labels on Rpo4/7 were modeled using in vacuo molecular dynamics simulations (MD) and a stochastic Monte Carlo sampling approach. For the nitroxide probes we also performed MD simulations with explicit water and carried out a rotamer library analysis. Our results show that the Monte Carlo simulations are in better agreement with experiments than the MD simulations and the rotamer library approach results in plausible distance predictions. Because the latter is the least computationally demanding of the methods we have explored, and is readily available to many researchers, it prevails as the method of choice for the interpretation of DEER distance distributions.
Conflict of interest statement
Figures



References
-
- Edidin M. Fluorescence resonance energy transfer: techniques for measuring molecular conformation and molecular proximity. Curr Prot Immunol Chapter 18: Unit 18.10. 2003. - PubMed
-
- Schiemann O, Prisner TF. Long-range distance determinations in biomacromolecules by EPR spectroscopy. Q Rev Biophys. 2007;40:1–53. - PubMed
-
- Bordignon E, Steinhoff HJ. Membrane protein structure and dynamics studied by site-directed spin labeling ESR. In ESR Spectroscopy in Membrane Biophysics, Hemminga, M. A., Berliner, L. J., Eds.; New York: Springer Science and Business Media. Pp. 2007. pp. 129–164.
-
- Förster T. Transfer mechanisms of electronic excitation. Discuss Faraday Soc. 1959;27:7–17.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

