Sec5 and Exo84 mediate distinct aspects of RalA-dependent cell polarization
- PMID: 22761837
- PMCID: PMC3382198
- DOI: 10.1371/journal.pone.0039602
Sec5 and Exo84 mediate distinct aspects of RalA-dependent cell polarization
Abstract
Metastasis is a complex process during which several gross cellular changes occur. Cells must dissociate from the tumor mass and gain the ability to degrade extracellular matrix and migrate in order to ultimately attach and form a satellite tumor. Regulation of the actin cytoskeleton is an indispensible aspect of cell migration, and many different factors have been implicated in this process. We identified interactions between RalA and its effectors in the Exocyst complex as directly necessary for migration and invasion of prostate cancer tumor cells. Blocking RalA-Exocyst binding caused significant morphological changes and defects in single and coordinated cell migration.
Conflict of interest statement
Figures



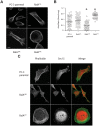

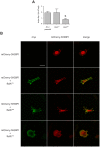
Similar articles
-
RalBP1 is necessary for metastasis of human cancer cell lines.Neoplasia. 2010 Dec;12(12):1003-12. doi: 10.1593/neo.101080. Neoplasia. 2010. PMID: 21170262 Free PMC article.
-
Ral-regulated interaction between Sec5 and paxillin targets Exocyst to focal complexes during cell migration.J Cell Sci. 2008 Sep 1;121(Pt 17):2880-91. doi: 10.1242/jcs.031641. Epub 2008 Aug 12. J Cell Sci. 2008. PMID: 18697830 Free PMC article.
-
RalA and the exocyst complex influence neuronal polarity through PAR-3 and aPKC.J Cell Sci. 2009 May 15;122(Pt 10):1499-506. doi: 10.1242/jcs.044339. Epub 2009 Apr 21. J Cell Sci. 2009. PMID: 19383721
-
The exocyst complex in exocytosis and cell migration.Protoplasma. 2012 Jul;249(3):587-97. doi: 10.1007/s00709-011-0330-1. Epub 2011 Oct 14. Protoplasma. 2012. PMID: 21997494 Review.
-
The role of Exo70 in exocytosis and beyond.Small GTPases. 2019 Sep;10(5):331-335. doi: 10.1080/21541248.2017.1328998. Epub 2017 Jun 19. Small GTPases. 2019. PMID: 28489961 Free PMC article. Review.
Cited by
-
Human and mouse microarrays-guided expression analysis of membrane protein trafficking-related genes in MDCK cells, a canine epithelial model for apical and basolateral differential protein targeting.Biochim Open. 2017 Apr 30;4:119-126. doi: 10.1016/j.biopen.2017.04.002. eCollection 2017 Jun. Biochim Open. 2017. PMID: 29450149 Free PMC article.
-
Characterization of somatic mutations and pathway alterations during hepatocellular carcinoma vascular invasion using next-generation sequencing.J Gastrointest Oncol. 2022 Aug;13(4):1864-1874. doi: 10.21037/jgo-22-556. J Gastrointest Oncol. 2022. PMID: 36092348 Free PMC article.
-
Functional impact of multi-omic interactions in breast cancer subtypes.Front Genet. 2023 Jan 5;13:1078609. doi: 10.3389/fgene.2022.1078609. eCollection 2022. Front Genet. 2023. PMID: 36685900 Free PMC article.
-
Phosphatidic Acid Produced by RalA-activated PLD2 Stimulates Caveolae-mediated Endocytosis and Trafficking in Endothelial Cells.J Biol Chem. 2016 Sep 23;291(39):20729-38. doi: 10.1074/jbc.M116.752485. Epub 2016 Aug 10. J Biol Chem. 2016. PMID: 27510034 Free PMC article.
-
The RAL Enigma: Distinct Roles of RALA and RALB in Cancer.Cells. 2022 May 14;11(10):1645. doi: 10.3390/cells11101645. Cells. 2022. PMID: 35626682 Free PMC article. Review.
References
-
- Oka H, Shiozaki H, Kobayashi K, Inoue M, Tahara H, et al. Expression of E-cadherin cell adhesion molecules in human breast cancer tissues and its relationship to metastasis. Cancer research. 1993;53:1696–1701. - PubMed
-
- Onder TT, Gupta PB, Mani SA, Yang J, Lander ES, et al. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer research. 2008;68:3645–3654. - PubMed
-
- Ridley AJ. Rho GTPases and cell migration. Journal of cell science. 2001;114:2713–2722. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

