Myoferlin depletion in breast cancer cells promotes mesenchymal to epithelial shape change and stalls invasion
- PMID: 22761893
- PMCID: PMC3384637
- DOI: 10.1371/journal.pone.0039766
Myoferlin depletion in breast cancer cells promotes mesenchymal to epithelial shape change and stalls invasion
Abstract
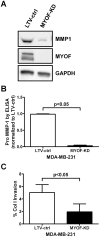
Myoferlin (MYOF) is a mammalian ferlin protein with homology to ancestral Fer-1, a nematode protein that regulates spermatic membrane fusion, which underlies the amoeboid-like movements of its sperm. Studies in muscle and endothelial cells have reported on the role of myoferlin in membrane repair, endocytosis, myoblast fusion, and the proper expression of various plasma membrane receptors. In this study, using an in vitro human breast cancer cell model, we demonstrate that myoferlin is abundantly expressed in invasive breast tumor cells. Depletion of MYOF using lentiviral-driven shRNA expression revealed that MDA-MB-231 cells reverted to an epithelial morphology, suggesting at least some features of mesenchymal to epithelial transition (MET). These observations were confirmed by the down-regulation of some mesenchymal cell markers (e.g., fibronectin and vimentin) and coordinate up-regulation of the E-cadherin epithelial marker. Cell invasion assays using Boyden chambers showed that loss of MYOF led to a significant diminution in invasion through Matrigel or type I collagen, while cell migration was unaffected. PCR array and screening of serum-free culture supernatants from shRNA(MYOF) transduced MDA-MB-231 cells indicated a significant reduction in the steady-state levels of several matrix metalloproteinases. These data when considered in toto suggest a novel role of MYOF in breast tumor cell invasion and a potential reversion to an epithelial phenotype upon loss of MYOF.
Conflict of interest statement
Figures





References
-
- American Cancer Society. Breast Cancer Facts & Figures 2009–2010. 2009.
-
- Horner MRL, Krapcho M, Neyman N, Aminou R, Howlader N, et al. SEER Cancer Statistics Review, 1975–2006. National Cancer Institute. National Cancer Institute Surveillance Epidemiology and End Results website. 2009. Available: http://seer.cancer.gov/csr/1975_2006/. Accessed 2012 Jan 12.
-
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. - PubMed
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. - PubMed
-
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

