β-Catenin loss in hepatocytes promotes hepatocellular cancer after diethylnitrosamine and phenobarbital administration to mice
- PMID: 22761897
- PMCID: PMC3382575
- DOI: 10.1371/journal.pone.0039771
β-Catenin loss in hepatocytes promotes hepatocellular cancer after diethylnitrosamine and phenobarbital administration to mice
Abstract
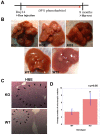
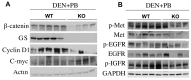
Hepatocellular Carcinoma (HCC) is the fifth most common cancer worldwide. β-Catenin, the central orchestrator of the canonical Wnt pathway and a known oncogene is paramount in HCC pathogenesis. Administration of phenobarbital (PB) containing water (0.05% w/v) as tumor promoter following initial injected intraperitoneal (IP) diethylnitrosamine (DEN) injection (5 µg/gm body weight) as a tumor inducer is commonly used model to study HCC in mice. Herein, nine fifteen-day male β-catenin knockout mice (KO) and fifteen wild-type littermate controls (WT) underwent DEN/PB treatment and were examined for hepatic tumorigenesis at eight months. Paradoxically, a significantly higher tumor burden was observed in KO (p<0.05). Tumors in KO were β-catenin and glutamine synthetase negative and HGF/Met, EGFR & IGFR signaling was unremarkable. A significant increase in PDGFRα and its ligand PDGF-CC leading to increased phosphotyrosine-720-PDGFRα was observed in tumor-bearing KO mice (p<0.05). Simultaneously, these livers displayed increased cell death, stellate cell activation, hepatic fibrosis and cell proliferation. Further, PDGF-CC significantly induced hepatoma cell proliferation especially following β-catenin suppression. Our studies also demonstrate that the utilized DEN/PB protocol in the WT C57BL/6 mice did not select for β-catenin gene mutations during hepatocarcinogenesis. Thus, DEN/PB enhanced HCC in mice lacking β-catenin in the liver may be due to their ineptness at regulating cell survival, leading to enhanced fibrosis and regeneration through PDGFRα activation. β-Catenin downregulation also made hepatoma cells more sensitive to receptor tyrosine kinases and thus may be exploited for therapeutics.
Conflict of interest statement
Figures




Comment in
-
The connection of β-catenin and phenobarbital in murine hepatocarcinogenesis: a critical discussion of Awuah et al., PLoS ONE 7(6):e39771, 2012.Arch Toxicol. 2013 Mar;87(3):401-2. doi: 10.1007/s00204-012-1002-4. Epub 2012 Dec 25. Arch Toxicol. 2013. PMID: 23266721 No abstract available.
References
-
- Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2:533–543. - PubMed
-
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. - PubMed
-
- Peifer M, Polakis P. Wnt signaling in oncogenesis and embryogenesis–a look outside the nucleus. Science. 2000;287:1606–1609. - PubMed
-
- Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

