Epigenetic effects of prenatal stress on 11β-hydroxysteroid dehydrogenase-2 in the placenta and fetal brain
- PMID: 22761903
- PMCID: PMC3383683
- DOI: 10.1371/journal.pone.0039791
Epigenetic effects of prenatal stress on 11β-hydroxysteroid dehydrogenase-2 in the placenta and fetal brain
Abstract
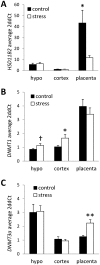
Maternal exposure to stress during pregnancy is associated with significant alterations in offspring neurodevelopment and elevated maternal glucocorticoids likely play a central role in mediating these effects. Placental 11β-hydroxysteroid dehydrogenase type 2 (HSD11B2) buffers the impact of maternal glucocorticoid exposure by converting cortisol/corticosterone into inactive metabolites. However, previous studies indicate that maternal adversity during the prenatal period can lead to a down-regulation of this enzyme. In the current study, we examined the impact of prenatal stress (chronic restraint stress during gestational days 14-20) in Long Evans rats on HSD11B2 mRNA in the placenta and fetal brain (E20) and assessed the role of epigenetic mechanisms in these stress-induced effects. In the placenta, prenatal stress was associated with a significant decrease in HSD11B2 mRNA, increased mRNA levels of the DNA methyltransferase DNMT3a, and increased DNA methylation at specific CpG sites within the HSD11B2 gene promoter. Within the fetal hypothalamus, though we find no stress-induced effects on HSD11B2 mRNA levels, prenatal stress induced decreased CpG methylation within the HSD11B2 promoter and increased methylation at sites within exon 1. Within the fetal cortex, HSD11B2 mRNA and DNA methylation levels were not altered by prenatal stress, though we did find stress-induced elevations in DNMT1 mRNA in this brain region. Within individuals, we identified CpG sites within the HSD11B2 gene promoter and exon 1 at which DNA methylation levels were highly correlated between the placenta and fetal cortex. Overall, our findings implicate DNA methylation as a mechanism by which prenatal stress alters HSD11B2 gene expression. These findings highlight the tissue specificity of epigenetic effects, but also raise the intriguing possibility of using the epigenetic status of placenta to predict corresponding changes in the brain.
Conflict of interest statement
Figures

References
-
- Dancause KN, Laplante DP, Oremus C, Fraser S, Brunet A, et al. Disaster-related prenatal maternal stress influences birth outcomes: project Ice Storm. Early Hum Dev. 2011;87:813–820. - PubMed
-
- Hobel CJ, Goldstein A, Barrett ES. Psychosocial stress and pregnancy outcome. Clin Obstet Gynecol. 2008;51:333–348. - PubMed
-
- Wadhwa PD, Garite TJ, Porto M, Glynn L, Chicz-DeMet A, et al. Placental corticotropin-releasing hormone (CRH), spontaneous preterm birth, and fetal growth restriction: a prospective investigation. Am J Obstet Gynecol. 2004;191:1063–1069. - PubMed
-
- O’Connor TG, Heron J, Golding J, Beveridge M, Glover V. Maternal antenatal anxiety and children's behavioural/emotional problems at 4 years. Report from the Avon Longitudinal Study of Parents and Children. Br J Psychiatry. 2002;180:502–508. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

