Characterization of the autocrine/paracrine function of vitamin D in human gingival fibroblasts and periodontal ligament cells
- PMID: 22761920
- PMCID: PMC3382579
- DOI: 10.1371/journal.pone.0039878
Characterization of the autocrine/paracrine function of vitamin D in human gingival fibroblasts and periodontal ligament cells
Abstract
Background: We previously demonstrated that 25-hydroxyvitamin D(3), the precursor of 1α,25-dihydroxyvitamin D(3), is abundant around periodontal soft tissues. Here we investigate whether 25-hydroxyvitamin D(3) is converted to 1α,25-dihydroxyvitamin D(3) in periodontal soft tissue cells and explore the possibility of an autocrine/paracrine function of 1α,25-dihydroxyvitamin D(3) in periodontal soft tissue cells.
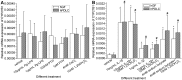
Methodology/principal findings: We established primary cultures of human gingival fibroblasts and human periodontal ligament cells from 5 individual donors. We demonstrated that 1α-hydroxylase was expressed in human gingival fibroblasts and periodontal ligament cells, as was cubilin. After incubation with the 1α-hydroxylase substrate 25-hydroxyvitamin D(3), human gingival fibroblasts and periodontal ligament cells generated detectable 1α,25-dihydroxyvitamin D(3) that resulted in an up-regulation of CYP24A1 and RANKL mRNA. A specific knockdown of 1α-hydroxylase in human gingival fibroblasts and periodontal ligament cells using siRNA resulted in a significant reduction in both 1α,25-dihydroxyvitamin D(3) production and mRNA expression of CYP24A1 and RANKL. The classical renal regulators of 1α-hydroxylase (parathyroid hormone, calcium and 1α,25-dihydroxyvitamin D(3)) and Porphyromonas gingivalis lipopolysaccharide did not influence 1α-hydroxylase expression significantly, however, interleukin-1β and sodium butyrate strongly induced 1α-hydroxylase expression in human gingival fibroblasts and periodontal ligament cells.
Conclusions/significance: In this study, the expression, activity and functionality of 1α-hydroxylase were detected in human gingival fibroblasts and periodontal ligament cells, raising the possibility that vitamin D acts in an autocrine/paracrine manner in these cells.
Conflict of interest statement
Figures








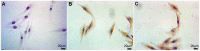
References
-
- Rachez C, Freedman LP. Mechanisms of gene regulation by vitamin D(3) receptor: a network of coactivator interactions. Gene. 2000;246:9–21. - PubMed
-
- von EM, Kongsbak M, Schjerling P, Olgaard K, Odum N, et al. Vitamin D controls T cell antigen receptor signaling and activation of human T cells. Nat Immunol. 2010;11:344–349. - PubMed
-
- Chen KS, DeLuca HF. Cloning of the human 1 alpha,25-dihydroxyvitamin D-3 24-hydroxylase gene promoter and identification of two vitamin D-responsive elements. Biochim Biophys Acta. 1995;1263:1–9. - PubMed
-
- Haussler MR, Whitfield GK, Haussler CA, Hsieh JC, Thompson PD, et al. The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J Bone Miner Res. 1998;13:325–349. - PubMed
-
- Christakos S, Dhawan P, Liu Y, Peng X, Porta A. New insights into the mechanisms of vitamin D action. J Cell Biochem. 2003;88:695–705. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

